Mineral and Energy Resources
Mineral and Energy Resources
back to Contents of Entire Course...
Mineral Resources
Origin of Mineral Deposits
Mineral Deposits and Plate
Tectonics
Energy Resources
Solar Energy
Nuclear Energy
Fossil Fuels
Formation of Petroleum
Oil Traps
Distribution of Petroleum
Oil Shale and Tar Sands
Coal
adapted to HTML from lecture notes of Prof. Stephen A. Nelson Tulane
University
Mineral
Resources
Almost all Earth Materials are used by humans for something. We require
metals for making machines, sands and gravels for making roads and
buildings, sand
for making computer chips, limestone and gypsum for making concrete,
clays for making ceramics, gold, silver, copper and aluminum for making
electric circuits, and diamonds
and corundum (sapphire,
ruby, emerald) for abrasives and jewelry. A mineral deposit is a
volume of rock enriched in one or more materials. In this sense a mineral
refers to a useful material, a definition that is different from the way
we defined a mineral back in Chapter 2. Here the word mineral can be any
substance that comes from the Earth. Finding and exploiting mineral
deposits requires the application of the principles of geology that you
have learned throughout this course. Some minerals are used as they are
found in the ground, i.e. they require no further processing or very
little processing. For example - gemstones, sand, gravel, and salt
(halite). Most minerals must be processed before they are used. For
example:
- Iron is the found in abundance in minerals, but the process of extracting iron from different minerals varies in cost depending on the mineral. It is least costly to extract the iron from oxide minerals like hematite (Fe2O3), magnetite (Fe3O4), or limonite [Fe(OH)]. Although iron also occurs in olivines, pyroxenes, amphiboles, and biotite, the concentration of iron in these minerals is less, and cost of extraction is increased because strong bonds between iron, silicon, and oxygen must be broken.
- Aluminum is the third most abundant mineral in the Earth's crust. It occurs in the most common minerals of the crust - the feldspars (NaAlSi3O8, KalSi3O8, & CaAl2Si2O8, but the cost of extracting the Aluminum from these minerals is high. Thus, deposits containing the mineral gibbsite [Al(OH)3], are usually sought. This explains why recycling of Aluminum is cost effective, since the Aluminum does not have to be separated from oxygen or silicon.
Because such things as extraction costs, manpower costs, and energy costs vary with time and from country to country, what constitutes an economically viable deposit of minerals varies considerably in time and place. In general, the higher the concentration of the substance, the more economical it is to mine. Thus we define an ore as a mineral deposit from which one or more valuable substances can be extracted economically. An ore deposit will consist of ore minerals, that contain the valuable substance.
Gangue minerals are minerals that occur in the deposit but do not contain the valuable substance
Since economics is what controls the grade or concentration of the substance in a deposit that makes the deposit profitable to mine, different substances require different concentrations to be profitable.. But, the concentration that can be economically mined changes due to economic conditions such as demand for the substance and the cost of extraction.
Examples:
- The copper concentration in copper ore deposits has shown changes throughout history. From 1880 to about 1960 the grade of copper ore showed a steady decrease from about 3% to less than 1%, mainly due to increased efficiency of mining. From about 1960 to 1980 the grade increased to over 1% due to increasing costs of energy and an abundant supply produced by cheaper labor in other countries.
- Gold prices vary on a daily basis. When gold prices are high, old abandoned mines re-open, when the price drops, gold mines close. The cost of labor is currently so high in the U.S. that few gold mines can operate profitably, but in third world countries where labor costs are lower, gold mines that have ore concentrations well below those found in the U.S. can operate with a profit.
| Substance | Average Crustal Abundance | Concentration Factor |
|---|---|---|
| Al (Aluminum) | 8.0% | 3 to 4 |
| Fe (Iron) | 5.8% | 6 to7 |
| Ti (Titanium) | 0.86% | 25 to 100 |
| Cr (Chromium) | 0.0096% | 4,000 to 5,000 |
| Zn (Zinc) | 0.0082% | 300 |
| Cu (Copper) | 0.0058% | 100 to 200 |
| Ag (Silver) | 0.000008% | ~1000 |
| Pt (Platinum) | 0.0000005% | 600 |
| Au (Gold) | 0.0000002% | 4,000 to 5,000 |
| U (Uranium) | 0.00016% | 500 to 1000 |
Note that we will not likely ever run out of a useful substance, since we can always find deposits of any substance that have lower concentrations than are currently economical. If the supply of currently economical deposits is reduced, the price will increase and the concentration factor will increase
Origin of Mineral Deposits
Mineral deposits can be classified on the basis of the mechanism responsible for concentrating the valuable substance.
Hydrothermal Mineral Deposits
- Concentration by hot aqueous (water-rich) fluids flowing through fractures and pore spaces in rocks.Hydrothermal deposits are produced when groundwater circulates to depth and heats up either by coming near a hot igneous body at depth or by circulating to great depth along the geothermal gradient. Such hot water can dissolve valuable substances throughout a large volume of rock. As the hot water moves into cooler areas of the crust, the dissolved substances are precipitated from the hot water solution. If the cooling takes place rapidly, such as might occur in open fractures or upon reaching a body of cool surface water, then precipitation will take place over a limited area, resulting in a concentration of the substance attaining a higher value than was originally present in the rocks through which the water passed.
Examples:
- Massive sulfide deposits at oceanic spreading centers. Hot fluids circulating above the magma chambers at oceanic ridges can scavenge elements like Sulfur, Copper, and Zinc from the rocks through which they pass. As these hot fluids migrate back toward the seafloor, they come in contact with cold groundwater or sea water and suddenly precipitate these metals as sulfide minerals like sphalerite (zinc sulfide) and chalcopyrite (Copper, Iron sulfide).
- Vein deposits surrounding igneous intrusions. Hot water circulating around igneous intrusions scavenges metals and silica from both the intrusions and the surrounding rock. When these fluids are injected into open fractures, they cool rapidly and precipitate mainly quartz, but also a variety of sulfide minerals, and sometimes gold, and silver within the veins of quartz. Rich deposits of copper, zinc, lead, gold, silver, tin, mercury, and molybdenum result.
- Stratabound mineral deposits in lake or oceanic sediments. When hot groundwater containing valuable metals scavenged along their flow paths enters unconsolidated sediments on the bottom of a lake or ocean, it may precipitate ore minerals in the pore spaces between grains in the sediment. Such minerals may contain high concentrations of lead, zinc, and copper, usually in sulfide minerals like galena (lead sulfide), sphalerite (zinc sulfide), and chalcopyrite (copper-iron sulfide). Since they are included within the sedimentary strata they are called stratabound mineral deposits.
Magmatic Mineral Deposits
- substances are concentrated within a body of igneous rock by magmatic processes like crystal fractionation and crystal settling.
Magmatic process such as partial melting, crystal fractionation, or crystal settling in a magma chamber can concentrate ore minerals containing valuable substances by taking elements that were once widely dispersed in low concentrations in the magma and concentrating them in minerals that separate from the magma.
Examples:
- Pegmatites - During fractional crystallization water and elements that do not enter the minerals separated from the magma by crystallization will end up as the last residue of the original magma. This residue is rich in silica and water along with elements like the Rare Earth Elements (many of which are important for making phosphors in color television picture tubes), Lithium, Tantalum, Niobium, Boron, Beryllium, Gold, and Uranium. This residue is often injected into fractures surrounding the igneous intrusion and crystallizes as a rock called a pegmatite that characteristically consists of large crystals.
- Crystal Settling. As minerals crystallize from a magma body, heavy minerals may sink to the bottom of the magma chamber. Such heavy minerals as chromite, olivine, and ilmenite contain high concentrations of Chromium, Titanium, Platinum, Nickel, and Iron. These elements thus attain higher concentrations in the layers that form on the bottom of the magma chamber
Sedimentary Mineral Deposits
- substances are concentrated by chemical precipitation from lake or sea water.
Although clastic sedimentary processes can form mineral deposits, the term sedimentary mineral deposit is restricted to chemical sedimentation, where minerals containing valuable substances are precipitated directly out of water.
Examples:
- Evaporite Deposits - Evaporation of lake water or sea water results in the loss of water and thus concentrates dissolved substances in the remaining water. When the water becomes saturated in such dissolved substance they precipitate from the water. Deposits of halite (table salt), gypsum (used in plaster and wall board), borax (used in soap), and sylvite (potassium chloride, from which potassium is extracted to use in fertilizers) result from this process.
- Iron Formations - These deposits are of iron rich chert and a number of other iron bearing minerals that were deposited in basins within continental crust during the Proterozoic (2 billion years or older). They appear to be evaporite type deposits, but if so, the composition of sea water must have been drastically different than it is today
- Placer
Mineral Deposits - substances are concentrated by flowing
surface waters either in streams or along coastlines.
The velocity of flowing water determines whether minerals are carried in suspension or deposited. When the velocity of the water slows, large minerals or minerals with a higher density are deposited. Heavy minerals like gold, diamond, and magnetite of the same size as a low density mineral like quartz will be deposited at a higher velocity than the quartz, thus the heavy minerals will be concentrated in areas where water current velocity is low. Mineral deposits formed in this way are called placer deposits. They occur in any area where current velocity is low, such as in point bar deposits, between ripple marks, behind submerged bars, or in holes on the bottom of a stream. The California gold rush in 1849 began when someone discovered rich placer deposits of gold in streams draining the Sierra Nevada Mountains. The gold originally formed in hydrothermal veins, but it was eroded out of the veins and carried in streams where it was deposited in placer deposits.
Residual Mineral Deposits
- substances are concentrated by chemical weathering processes.During chemical weathering and original body of rock is greatly reduced in volume by the process of leaching, which removes ions from the original rock. Elements that are not leached form the rock thus occur in higher concentration in the residual rock. The most important ore of Aluminum, bauxite, forms in tropical climates where high temperatures and high water throughput during chemical weathering produces highly leached lateritic soils rich in both iron and aluminum. Most bauxite deposits are relatively young because they form near the surface of the Earth and are easily removed by erosion acting over long periods of time. In addition, an existing mineral deposit can be turned in to a more highly concentrated mineral deposit by weathering in a process called secondary enrichment
Mineral Deposits and Plate Tectonics
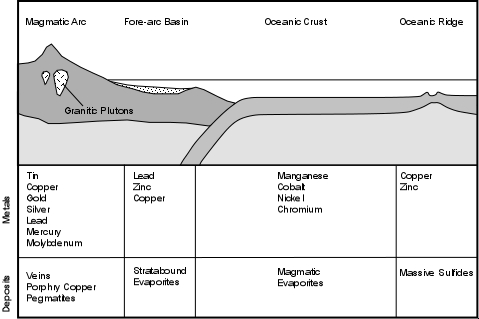
Because different types of mineral deposits form in different environments, plate tectonics plays a critical role in the location of different geological environments. The diagram to the right shows the different mineral deposits that occur in different tectonic environments.
Energy Resources
Energy reaches the surface of the Earth from two main sources, and thus allows us to divide energy into two types.
- Solar Energy - which arrives at the Earth as electromagnetic radiation from the Sun.
- Nuclear Energy - which is produced within the Earth by radioactive decay of atoms.
In reality, both types are nuclear energy because they involve the energy that holds atoms together. For the purposes of this course we will classify energy resources into these two groups and further subdivide each group into the basic energy sources that can be exploited by human beings.
Solar Energy
- Energy derived directly from the Sun
- Direct solar energy - used to heat water and homes - can be used to generate electricity with solar cells*.
- Wind Energy - Solar energy causes heating of the atmosphere that results in convection of air and produces winds.
- Hydroelectric Energy* - derived from the Sun because the Sun causes evaporation of the oceans. Convection of the atmosphere moves the evaporated water to higher elevations where it can then run down hill and be used to generate electricity.
- Energy derived indirectly from the Sun
- Biomass Energy - involves burning of wood, or other organic byproducts. Such organic material is produced by photosynthesis, a chemical process which derives energy from the Sun and stores that energy until the material is burned.
- Fossil Fuels* - Biomass energy that is buried within the Earth where it is stored until humans extract and burn it to release the energy.
- Petroleum (Oil & natural gas), oil shale, and tar sands.
- Coal
Nuclear Energy
Geothermal Energy* - Decay of radioactive elements has produced heat throughout Earth history. It is this heat that causes the temperature to increase with depth in the Earth and is responsible for melting of mantle rocks to form magmas. Magmas can carry this upward into the crust. Groundwater circulating in the vicinity of igneous intrusions carries the heat back toward the surface. If this hot water can be tapped, it can be used directly to heat homes, or if trapped at great depth under pressure it can be turned into steam which will expand and drive a turbine to generate electricity.
Direct Nuclear Energy* - Radioactive Uranium is concentrated and made into fuel rods that generate large amounts of heat as a result of radioactive decay. This heat is used to turn water into steam. Expansion of the steam can then be used to drive a turbine and generate electricity. Once proposed as a cheap, clean, and safe way to generate energy, Nuclear power has come under some disfavor. Costs of making sure nuclear power plants are clean and safe and the problem of disposing of radioactive wastes, which are unsafe, as well as questions about the safety of the plants under human care, have contributed to this disfavour In the above list, sources of energy that require geological knowledge for exploitation are marked with an asterisk (*). While using direct solar energy to heat water and homes does not require geologic knowledge, the making of solar cells does, because the material to make such cells requires knowledge of specific mineral deposits. Hydroelectric energy requires geologic knowledge in order to make sure that dams are built in areas where they will not collapse and harm human populations. Finding fossil fuels and geothermal energy certainly requires geologic knowledge. Direct nuclear energy requires geologists to find deposits of uranium to generate the fuels, geologists to find sites for nuclear power plants that will not fall apart due to such things as earthquakes, landslides, floods, or volcanic eruptions, and requires geologists to help determine safe storage sites for nuclear waste products. In this course we will concentrate on Fossil Fuels.
Fossil Fuels
The origin of fossil fuels, and biomass energy in general, starts with photosynthesis. Photosynthesis is the most important chemical reaction to us as human beings, because without it, we could not. Photosynthesis is the reaction that combines water and carbon dioxide from the Earth and its atmosphere with solar energy to form organic molecules that make up plants and oxygen essential for respiration. Because all life forms depend on plants for nourishment, either directly or indirectly, photosynthesis is the basis for life on Earth. The chemical reaction is so important, that everyone should know it as it is the way plants produce food from inorganic materials and is the basis for the vast majority of the food chain.
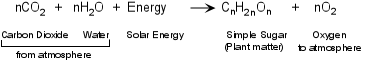
Note that if the reaction runs in reverse, it produces energy. Thus when oxygen is added to organic material, either through decay by reaction with oxygen in the atmosphere or by adding oxygen directly by burning, energy is produced, and water and carbon dioxide return to the Earth or its atmosphere. To produce a fossil fuel, the organic matter must be rapidly buried in the Earth so that it does not oxidize (react with oxygen in the atmosphere). Then a series of slow chemical reactions occur which turn the organic molecules into hydrocarbons. Hydrocarbons are complex organic molecules that consist of chains of hydrogen and carbon.
Petroleum (oil and natural gas) consists of many different such hydrocarbons, but the most important of these are a group known as the paraffins. Paraffins have the general chemical formula:
CnH2n+2
As the value of n in the formula increases, the following compounds are produced:
Formation of
Petroleum
The organic matter that eventually becomes petroleum is derived from photosynthetic microscopic organisms, like plankton and bacteria, originally deposited along with clays in the oceans. The resulting rocks are usually shales, yet, most petroleum occurs in much more permeable rocks like sandstones, limestones, or highly fractured rock. Thus, it is apparent that petroleum migrates, like groundwater, and accumulates in these more permeable rocks. The process of petroleum formation involves several steps:
- Organic matter from organisms must be produced in great abundance.
- This organic matter must be buried rapidly before oxidation takes place.
- Slow chemical reactions transform the organic material into the hydrocarbons found in petroleum.
As a result of compaction of the sediments containing the petroleum, the oil and natural gas are forced out and migrate into permeable rock. Migration is similar to groundwater flow.
- The petroleum must migrate into a reservoir rock that is in some way capped by impermeable rocks to prevent the petroleum from leaking out to the surface of the Earth. Such a geologic structure is called a trap.
All of these processes must occur within a specific range of temperatures and pressures. If higher pressures and temperatures are encountered as a result of metamorphism or igneous activity, the petroleum will be broken down to other non-useful forms of hydrogen and carbon
Oil Traps
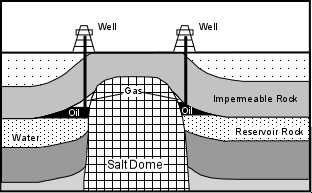
Because oil and natural gas have a low density they will migrate upward through the Earth and accumulate in a reservoir only if a geologic structure is present to trap the petroleum. Geologic structures wherein impermeable rocks occur above the permeable reservoir rock are required. The job of petroleum geologists searching for petroleum reservoirs, is to find conditions near the Earth's surface where such traps might occur. Oil traps can be divided into those that form as a result of geologic structures like folds and faults, called structural traps, and those that form as a result of stratigraphic relationships between rock units, called stratigraphic traps. If petroleum has migrated into a reservoir formed by one of these traps, note that the petroleum, like groundwater, will occur in the pore spaces of the rock. Natural gas will occur above the oil, which in turn will overly water in the pore spaces of the reservoir. This occurs because the density of natural gas is lower than that of oil, which is lower than that of water.
Structural Traps
Anticlines - If a permeable rocks like a sandstone or limestone is sandwiched between impermeable rock layers like shales or mudstones, and the rocks are folded into an anticline, petroleum can migrate upward in the permeable reservoir rocks, and will occur in the hinge region of the anticline
.
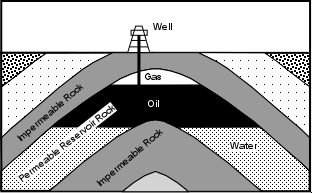
Since anticlines in the subsurface can often be found by looking at the orientation of rocks on the surface, anticlinal traps were among the first to be exploited by petroleum geologists. Note that synclines will not form an oil trap because oil must rise on top of the trapped water.
Faults - If faulting can juxtapose permeable and impermeable rocks so that the permeable rocks always have impermeable rocks above them, then an oil trap can form. Note that both normal faults and reverse faults can form this type of oil trap. Since faults are often exposed at the Earth's surface, the locations of such traps can often be found from surface exploration.
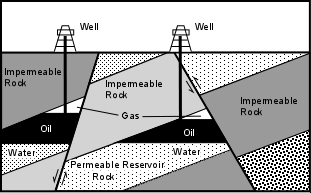
Salt Domes - During the Jurassic Period, the Gulf of Mexico was a restricted basin. This resulted in high evaporation rates & deposition of a thick layer of salt on the bottom of the basin. The salt was eventually covered with clastic sediments. But salt has a lower density than most sediments and is more ductile than most sedimentary rocks. Because of its low density, the salt moved upward through the sedimentary rocks as salt domes. The intrusion of the salt deforms the sedimentary strata along its margins, folding it upward to create oil traps. Because some salt domes get close to the surface, surface sediments overlying the salt dome are often domed upward, making the locations of the subsurface salt and possible oil traps easy to locate.
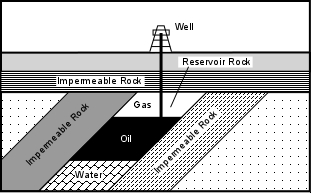
Stratigraphic Traps
Unconformities - An angular unconformity might form a suitable oil trap if the layers above the unconformity are impermeable rocks and permeable rocks layer are sandwiched between impermeable layers in the inclined strata below the unconformity. This type of trap is more difficult to locate because the unconformity may not be exposed at the Earth's surface
Locating possible traps like this usually requires subsurface exploration techniques, like drilling exploratory wells or using seismic waves to see what the structure looks like Lenses - Layers of sand often form lens like bodies that pinch out. If the rocks surrounding these lenses of sand are impermeable and deformation has produced inclined strata, oil and natural gas can migrate into the sand bodies and will be trapped by the impermeable rocks. This kind of trap is also difficult to locate from the surface, and requires subsurface exploration techniques
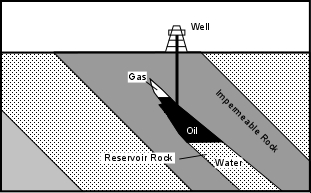
Distribution of Petroleum
The distribution of rocks containing petroleum widespread. Still, since reservoirs of petroleum result from upward migration and since older rocks have had more time to erode or metamorphose, most reservoirs of petroleum occur in younger rocks. Most petroleum is produced from rocks of Cenozoic age, with less produced from rocks of Mesozoic and Paleozoic age.

Oil Shale and Tar Sands
- Oil shale is shale that contains abundant organic matter that has not decomposed completely to produce petroleum. Oil can be extracted from oil shales, but they must be heated to high enough temperatures to drive the oil out. Since this process requires a lot of energy, exploitation of oil shales is not currently cost-effective, but may become so as other sources of petroleum become depleted. Known deposits of oil shale are extensive.
- Tar Sands are sandstones that have thick accumulations of viscous oil in their pore spaces. Extraction of this oil also requires heating the rock and is therefore energy intensive and not currently cost effective
Coal
Coal is a sedimentary/metanorphic rock produced in swamps where there is a large-scale accumulation of organic matter from plants. As the plants die they accumulate to first become peat. Compaction of the peat due to burial drives off volatile components like water and methane, eventually producing a black- colored organic- rich coal called lignite. Further compaction and heating results in a more carbon- rich coal called bituminous coal. If the rock becomes metamorphosed, a high grade coal called anthracite is produced. However, if temperatures and pressures become extremely high, all of the carbon is converted to graphite. Graphite will burn only at high temperatures and is therefore not useful as an energy source. Anthracite coal produces the most energy when burned, with less energy produced by bituminous coal and lignite. Coal is found in beds called seams, usually ranging in thickness from 0.5 to 3m, although some seams reach 30 m. Two major coal producing periods are known in geologic history. During the Carboniferous and Permian Periods, the continents were apparently located near the equator and covered by shallow seas. This type of environment favored the growth of vegetation and rapid burial to produce coal. Known reserves of coal far exceed those of other fossil fuels, and may be our best bet for an energy source of the future. Still, burning of the lower grades of coal, like lignite and bituminous coal produces large amounts of waste products that pollute the atmosphere. This problem needs to be overcome before we can further exploit this source of energy.

