Coal
Coal - types, formation, mining, "clean coal" electricity generation

Four kinds of coal
Two broad categories of coal
Coalification
Concept of Coal Rank
Types of underground mines
Coal Mining
Underground Coal Mining
Surface Coal Mining
Types of surface coal mines
"Clean coal"
electricity generation
The
difference between subcritical, supercritical and ultra-supercritical
boilers
Understanding
Australia’s emission reduction target
Carbon Capture and
Sequestration - CSS Technologies
"Clean"
Coal electricity generation and Carbon Capture and Storage - CCS
technologies Sources
Coal -
Uses, Formation, Affects - diagrams
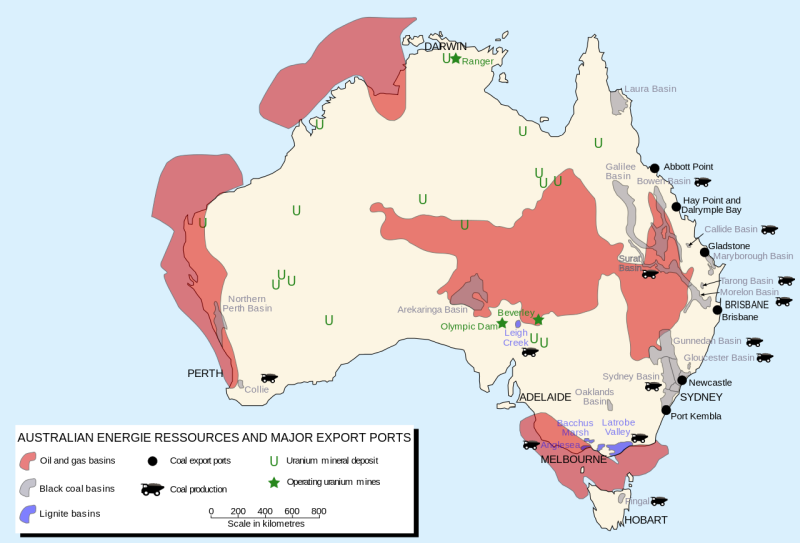
Four kinds of coal
Coal is a fossil fuel originating as plant material, compressed by geological pressures.The first evolutionary phase is peat which is little more than wood pulp that has been badly decomposed. There are large deposits of peat in the Scandinavian countries and Greenland. One can strip mine it since it is basically very close to the ground level. Strip mining is the process of scaping the coal from the top surface of the ground. The problem with peat is that it has a very low heat value per kilogram of the fuel burned. In addition, strip mining is ecologically very destructive unless the mining company makes a conscious effort to restore the country side.

The second phase in the evolutionary development of coal is lignite. Lignite is found in great quantities in the Western part of this country. Again lignite is not particularly efficient in producing energy per mass of fuel. There have been quite a bit of effort recently in the liquification and gasification of lignite. Liquification converts lignite into liquid crude petroleum. Gasification plants convert lignite into natural gas products. The conversion process is quite expensive, and with the present cost of other forms of fuel, it is economically infeasible. However, if other fuels become too expensive, this could be a more economical process. Other research has been conducted investigating other uses of lignite such as a fertilizer in hydroponic plant growth. Hydroponics is the use of nutrient containing water instead of soil in the growth of plant life.

A third phase in this coal development is soft coal (Bitumenous) which is one of the two stages used as a fuel in generating electrical power.
 Note bedding
Note beddingThe fourth and final phase results in the formation of hard coal (anthracite).

Two broad categories of coal
Coal is an organic sediment consisting of a complex mixture of substances.- Humic More common and originates from peat deposits consisting mostly of organic debris deposited in situ (autochthonous).
- Sapropelic Derived from redeposited (allochthonous) resistant
plant fragments such as spores or aquatic plants.
The sapropelic coals can be further subdivided into: - Cannel Coal Cannel coal is made up principally of uniformly sized plant fragments eg spores boghead coal Consists mainly of alginite (a coal maceral derived from algae).
- Peat is formed from the deposition of organic material with a restricted supply of oxygen. Peat forming environments are known generally as 'mires'.Mires may be classified as limnic or paralic...
- Limnic coal deposits formed inland in freshwater basins, peat bogs, or swamps. Peat forming environments isolated subsiding basins produce limnic coal deposits.
- Paralic deposit simply that there was a hydrological connection with the sea at the time of peat deposition. Mires may be found along coastal lowlands; as back barrier lagoons, estuaries and deltas.
Coalification
The transformation of plant material into coal takes place in two stages, biochemical degradation and physico-chemical degradation.Biochemical degradation involves chemical decomposition of botanical matter assisted by organisms.
- In tropical environments, this process may be faster, since the warm moist conditions are ideal for the organisms that assist in this process such as bacteria and fungi. However plant growth is also more rapid and so the increased rate of decomposition may be balanced by plant growth. In tropical conditions high rates of evaporation need to be coupled with high precipitation to maintain plant growth and peat accumulation.
- In cooler climates the growth rate of vegetation may be cyclical in nature and slower since the seasonal variation in conditions is greater. The conditions are less ideal for fungi and bacteria so the slower growth rate is matched by a slower rate of biochemical degradation.
Biochemical coalification ends at the rank of sub-bituminous coal, when humic substances have polymerised.
Physico-chemical coalification which follows is caused by conditions of burial.
The overburden which is deposited, the heat flows in the earth's crust and tectonic heat and pressure change the chemistry and structure of the altered organic material. The same conditions are applied to all the macerals.
Water is squeezed out and pore size is reduced as pressure increases and oxygen and hydrogen are released during thermal cracking. Water and carbon dioxide are the first products released.
When rank reaches medium volatile bituminous coal demethanation begins.
Concept of Coal Rank
The rank of a coal refers to the degree of coalification endured by the organic matter. It is estimated by measuring the moisture content, specific energy, reflectance of vitrinite or volatile matter (these are known as rank parameters). stages.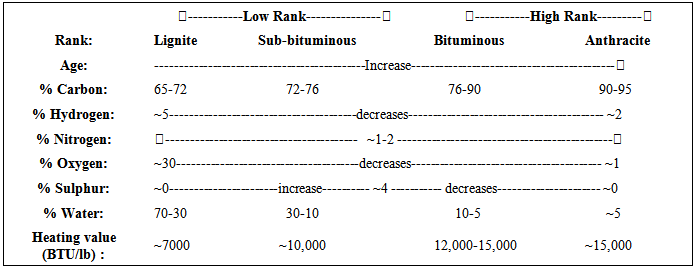
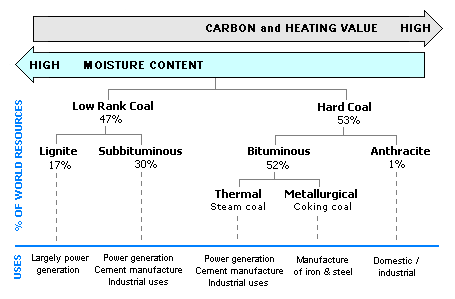
Coal uses based on rank
Coal Mining
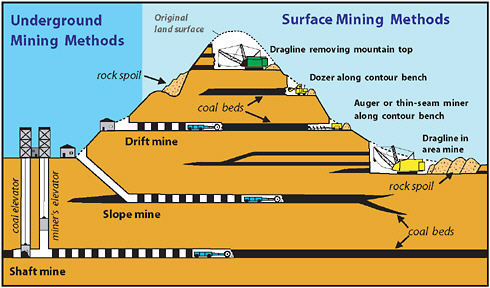
Underground Coal Mining
This drawing depicts the room and pillar method of underground mining.
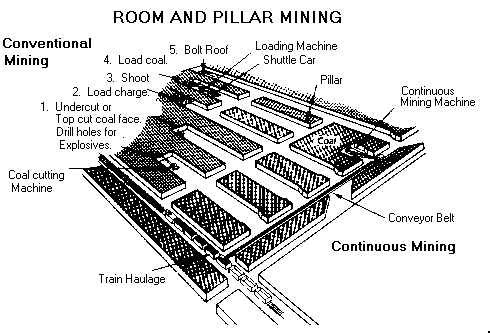
Most underground coal is mined by the room and pillar method, whereby rooms are cut into the coal bed leaving a series of pillars, or columns of coal, to help support the mine roof and control the flow of air. Generally, rooms are 630 metres wide and the pillars up to 100 metres wide. As mining advances, a grid-like pattern of rooms and pillars is formed. When mining advances to the end of a panel or the property line, retreat mining begins. In retreat mining, the workers mine as much coal as possible from the remaining pillars until the roof falls in. When retreat mining is completed, the mined area is abandoned.
There are two types of room and pillar mining--conventional mining and continuous mining
.
Conventional mining is the oldest method and accounts for only about 12% of underground coal output. In conventional mining, the coal seam is cut, drilled, blasted and then loaded into cars.
Continuous mining is the most prevalent form of underground mining, accounting for 56% of total underground production. In continuous mining, a machine known as a continuous miner cuts the coal from the mining face, obviating the need for drilling and blasting.
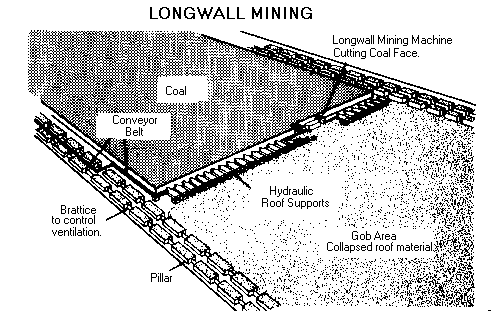
Types of underground mines--shaft mines, slope mines and drift mines.
The decision of what type of mine to construct depends on the depth of the coal seam and the surrounding terrain.
Drift mines have horizontal entries into the coal seam from a hillside.
Slope mines, which usually are not very deep, are inclined from the surface to the coal seam.
Shaft mines, generally the deepest mines, have vertical access to the coal seam via elevators that carry workers and equipment into the mine.
Almost all underground mines are less than 300 metres deep, but some mines reach depths of about 600 metres. miners in Nova Scotia actually mine coal beneath the ocean
\
Surface Coal Mining
 open pit mine
open pit mine open pit terms
open pit terms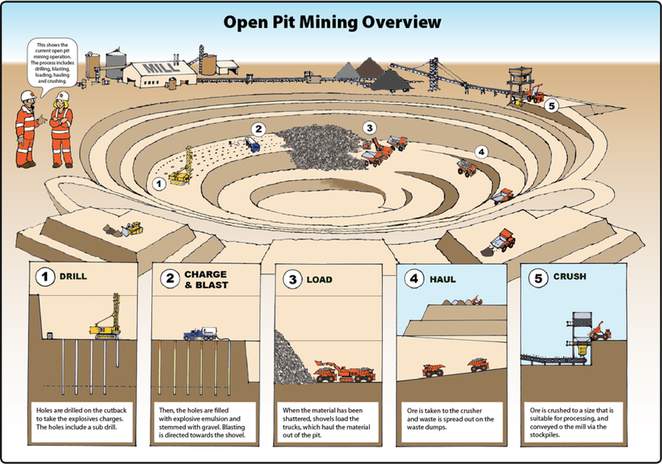 open pit
overview
open pit
overviewSurface mining is accomplished by removing overburden from the coal seam and then blasting and removing the coal. The ratio of overburden excavated to the amount of coal removed is called the overburden ratio. The lower the ratio, the more productive the mine. The lowest overburden ratios are found in western surface mines. , often more than one coal seam is mined.
Surface Mining Types
There are several types of surface coal mines.
Area surface mines , usually found in flat terrain, consist of a series of cuts 30 to 60 metres wide. The overburden from one cut is used to fill in the mined out area of the preceding cut.
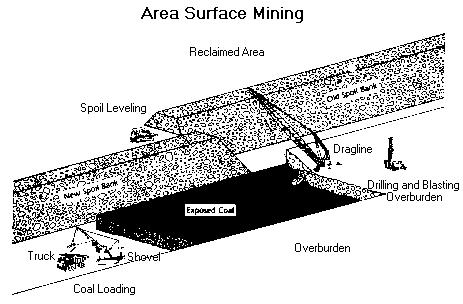
Contour mining , occurring in mountainous terrain, follows a coal seam along the side of the hill. When contour mining becomes too expensive, additional coal can often be produced from the mine's highwall by the use of augers or highwall miners.
Open pit mining is usually found where coal seams are thick. Open pit mines can reach depths of a hundred metres.
Equipment used in surface mines include draglines, shovels, bulldozers, front-end loaders, bucket wheel excavators and trucks. In large mines, draglines remove the overburden while shovels are used to load the coal. In smaller mines, bulldozers and front-end loaders are often used to remove overburden.
Clean coal electricity generation
Currently (late 2017) coal generation accounts for about 1/3 of Australia's carbon dioxide emissionsIs replacing existing coal generators with ultra-supercritical coal generation a reasonable strategy to reduce Australia's greenhouse gas emissions?
Australia’s coal generation fleet is ageing and needs replacing. Two-thirds of the 25 gigawatts in operation (after Victoria’s Hazelwood power station is retired in 2017) is more than 30 years old. By 2025 a further 18% of the fleet will be more than 30 years old.
That means that in 2025 a mere 4GW of our existing coal power will still be considered adequately efficient. This is important because efficient generation affects not only how much generators are paying for fuel, but also carbon dioxide (CO₂) emissions.
Modern coal power plants feed pulverised coal into a boiler to combust. Tubes in the boiler walls then absorb the heat and the steam generated in these boiler tubes turns the steam turbine and generates electricity.
The difference between subcritical, supercritical and ultra-supercritical boilers
The difference between subcritical, supercritical and ultra-supercritical boilers is in the steam conditions created in the boiler.- Supercritical and ultra-supercritical boilers are often referred to as high-efficiency, low-emissions technologies.
- Ultra-supercritical power stations are designed to operate at higher steam temperature and pressure. This improves efficiency, and has been made possible by new materials that can cope with higher temperatures.
- Ultra-supercritical coal power stations operate under steam conditions above 593-621℃ and a pressure of 28.4 million pascals
- Advanced ultra-supercritical plants are being considered
Comparisons of efficiencies...
- Sub-critical - Hazelwood converts only 22% of the gross energy, or calorific value, of coal into electricity. The remaining energy is lost as heat.
- Kogan Creek, currently Australia's most efficient coal burning power station is able to convert 37.5%
- Ultra-supercritical coal stations are able to convert up to 45% of the gross energy of coal to electricity, examples in Japan
- Advanced ultra-supercritical coal generation is expected to convert over 50% of the gross energy of coal to electricity, but the expensive alloys ( nickel-base alloys varying in chromium content from 20% to 25% ) required to accommodate the very high temperature requirements make the plants very expensive, in the vicinity of four times conventional cost At high temperatures and pressure water is very corrosive and can easily dissolve many common metals.
"which type of steam cycle is used has no impact on the emissions per tonne of coal burned"
What about Sulphur dioxide (SO2)?
Sulphur dioxide (SO2) emissions, emissions per tonne of coal depend solely on the amount of sulphur contained in the coal, essentially all of which is oxidized into SO2 during combustion, ending up in the raw flue gas.
For example, for typical “low-sulphur” coal containing 0.5 per cent of sulphur when fed into the boiler, every tonne of coal will contain 5 kilograms of sulphur. When burnt, this sulphur turns into 10 kilograms (kg) of SO2. (Every sulphur atom joins with two oxygen atoms to produce one SO2 molecule which is twice as heavy as a sulphur atom.)
The only difference between different steam cycles in terms of emissions is how much power they can generate from one tonne of coal.
Comparison of emission intensities...
- Hazelwood’s reported CO₂ emission intensity from 2014-15 was 1,400kg of greenhouse gas for every megawatt-hour of electricity it produced.
- Kogan Creek emitted 831kg per megawatt-hour.
- Ultra-supercritical generators can reduce emissions intensity to 760kg per megawatt-hour for black coal.
- Advanced ultra-supercritical generators can reduce emissions even further (maybe up to 50%?)
- Upgrading or replacing Victoria’s brown coal generators to ultra-supercritical would reduce emissions intensity to 928kg per megawatt-hour.
An example...
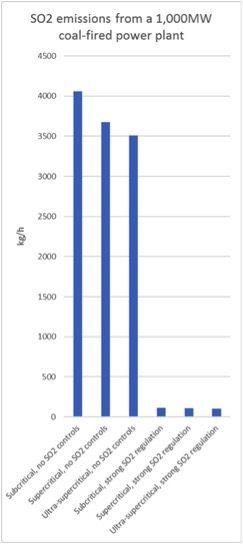
A 1000 megawatt (MW) coal-fired plant using subcritical technology will need to burn coal at a thermal input rate of 1000 MW / 38 per cent = 2630 MW-thermal to generate its full output. This corresponds to 410 tonnes of coal per hour, assuming a typical calorific value of 5500kcal/kg, and 4100kg/h of SO2 in raw flue gas.
If the plant uses ultra-supercritical technology, it needs thermal input of 1000 MW / 44 per cent = 2270 MW-thermal. As a result, it burns 350 tonnes of coal per hour, or 14 per cent less than the subcritical plant and generates 14 per cent less SO2.
If the plant is not equipped with SO2 emission control technology, that’s the end of the story.
However, if the environmental regulators require the plant to meet SO2 emission limits that cannot be met without installing SO2 control devices, the plant will have to make additional investments.
Cleaning the sulphur dioxide (not a greenhouse gas but still a serious pollutant)
Coal contains sulphur which when burnt becomes sulphur dioxide an air pollutant.
SO2 in the atmosphere reacts with water forming sulfurous acid/sulfuric acid droplets.These acid droplets reflect sunlight back to space leading to a lower insolation.
The result is a lower temperature on earth. That's the reason why after huge volcanic eruptions there may be no real summer for several years.
At the same time the acid droplets (see... acid rain), on reaching the ground acidify liquid water, resulting in examples of lifeless lakes and acid damaged forests.
So capturing SO2 is important
In essentially all countries except the US, SO2 emission limits are set in terms of SO2 concentrations in flue gas. The project developer will have to design a control device that removes enough of the SO2 from the flue gas to get below the limits.
Some of the toughest limits for SO2 emissions are found in China, where flue gases from coal-fired power plants are not allowed to contain more than 35 milligrams of SO2 for every cubic meter of dry flue gas.
The untreated flue gas from the example plants above will contain about 1200mg/m3 of SO2. Therefore, the plants will have to install SO2 control devices that remove about 97.5 per cent of the SO2 contained in untreated flue gas.
The
difference between subcritical and ultra-supercritical technology is
that the total amount of flue gas emitted from the ultra-supercritical
plant is about 14 per cent smaller, and hence the capacity of the SO2
control device can be about 14 per cent lower, resulting in savings in
investment and operating costs. Resulting SO2 emissions associated with
a given emission standard will also be about 14 per cent lower.
The same logic applies to the emissions coal plant (NOx), particulate matter (PM), mercury and other heavy metals. The air quality and health impacts are directly proportional to emissions.
So emissions regulation matters a lot, whether a plant is
ultra-supercritical matters little.The same logic applies to the emissions coal plant (NOx), particulate matter (PM), mercury and other heavy metals. The air quality and health impacts are directly proportional to emissions.
Q. Why are the coal industry and its advocates always going on about ultra-supercritical coal plants and not about emissions regulation?
A. Ultra-supercritical plants are usually more profitable than subcritical plants, since they have lower fuel and other operating costs.
Australia, advocates “High Efficiency Low Emissions” (HELE) coal plants along with Japan, but hasn’t required flue gas desulphurisation equipment on its own coal plants, making them some of the dirtiest in the world.
Below is a simple graph illustrating the effect of emissions regulation versus type of steam cycle on SO2 emissions:
Will switching to “High Efficiency Low Emissions” (HELE) coal plants be enough?
The problem is just how much CO₂ emissions can be reduced. Emissions from coal power are the largest contributors to Australia’s total emissions. In 2013-4, coal generators emitted 151 million tonnes of greenhouse gas, generating 154 million kilowatt-hours of electricity. This is 29% of Australia’s total emissions in 2013-14 of around 523 million tonnes. (Transport contributed around 18% to total emissions.)Let’s assume the current fleet of power stations is operating at 80% capacity, considered to be an economic optimum for coal power. This would generate 176 gigawatt-hours of electricity and 165 million tonnes of emissions. This allows for a 14% increase in consumption of electricity by 2030, which is likely given projections of population and economic growth.
If we then replace the entire 25GW, both black and brown, with ultra-supercritical generation, according to the assumptions included in the Australian Power Generation Technology Report, emissions would total 139 million tonnes. This would represent a 16% reduction in coal emissions, but a mere 5% reduction in Australia’s total emissions in 2013-4.
And then we would have those ultra-supercritical power stations for the next 30-40 years, incapable of reducing our emissions further as global targets tighten.
If Australia were to wait until advanced ultra-supercritical coal power is tested and trialled, then we could speculate that emissions from coal generation could reduce by a further 10% to 124 million tonnes. This would be a more promising 25% reduction in coal emissions, but still only a 7.7% reduction in Australia’s total emissions.
Understanding Australia’s emission reduction target
Australia’s emission reduction target for 2030 is 26-28% below 2005 levels.Emissions in 2005 were 594 million tonnes. Australia’s climate target would require emissions to reach around 434 million tonnes in 2030, a reduction of 160 million tonnes.
If coal power stations were to reduce emissions by 26-40 million tonnes through a shift to ultra-supercritical generators, then Australia would still be a very long way from meeting its committed targets.

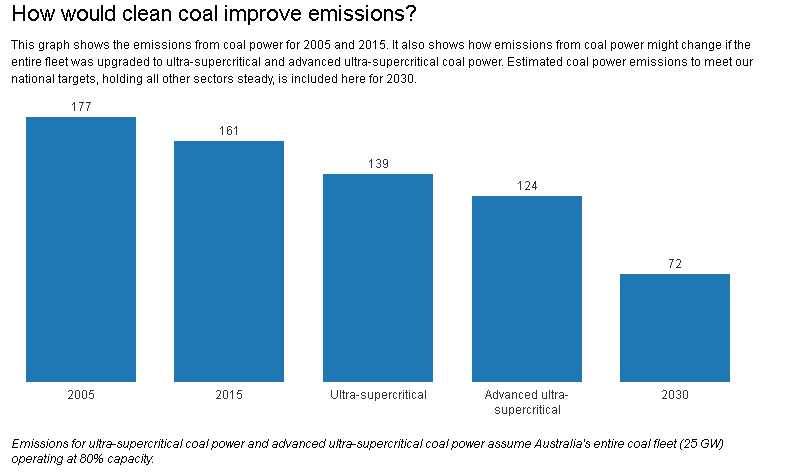
So the use of ultra-supercritical power generation would reduce our carbon emissions on their own but not by enough to meet our international obligations.
However what if we could just capture the carbon after it is released and store it somewhere? That could reduce our emissions further.
Carbon Capture and Storage - CCS technologies
The current range of CCS technologies can be grouped into “pre-combustion” and “post-combustion” methods.Carbon Capture...
- Pre-combustion methods typically react the carbon in the fuel with high-pressure steam to make hydrogen CO₂. The CO₂ is then separated (captured) from the hydrogen before the hydrogen is burned in the power station to make energy, with the only emissions being water vapour.
- Post-combustion technologies try to capture the carbon after it has been burned and becomes CO₂. If the fuel is burned in air, then the CO₂ needs to be separated from the exhaust gas stream which, like air, is mostly composed of nitrogen gas. This is usually done by passing the gas stream through a liquid that dissolves the CO₂ but not the nitrogen. Amine-based liquid absorbents, including ammonia, capture CO₂ from the flue gases before it is emitted to the atmosphere. The captured CO₂ product is pure enough that it is ready for compression, transport and storage. CSIRO has been working with industrial partners Delta Electricity and Stanwell Corporation, constructing and operating PCC pilot plants at Munmorah (New South Wales) and Tarong (Queensland) power stations to demonstrate carbon capture on actual flue gases during power production. (One of the advantages of PCC is that it can be retrofitted to existing power stations.) About 85% of the carbon dioxide present in the flue gas may be captured as a result of the chemical reaction between carbon dioxide and the absorbent. This process uses up about 30% of the electricity production.The carbon dioxide was released from the liquid absorbent by heating up the liquid absorbent to the point were the chemical reaction is reversed.So heat is needed to run the capture process. Solar thermal energy may be a possible heating source that reduces the additional electricity burden on the power station.
- Another technique - “Oxyfuelling”, has been developed in Australia. Oxyfuelling separates oxygen out of the air and then uses it to burn the fuel in an atmosphere of oxygen and recycled CO₂. The exhaust gas stream from this process is close enough to pure CO₂ that it can be sent directly to the storage process.
Several options have been explored for storing the carbon dioxide gas.
- injection of liquefied carbon dioxide into sedimentary rocks, the type of rocks in which oil and natural gas are found. Because oil and gas companies have so much experience working with these types of rocks, they are a natural place to store CO2 But these types of formations can only store the gas, not turn it into rock. With the volumes of CO2 produced it may be difficult to find enough safe host rock formations. And there is always a danger that the gas could escape to the atmosphere and add to global climate change.
- Similarly liquid CO2 is pumped to depth where the pressure of the overlying rocks prevents it from converting back to gas. CO2 leakage up a fault or joint into a populated area, could have catastrophic results. It is heavier that air and could suffocate people and animals. Lake Nyos is a crater lake in the Northwest Region of Cameroon that gave off periodic deadly bursts of CO2 that resulted in deaths.
- manufactured mineralised carbonate rock - (see... limestone CaCo3) - dropped into the deep ocean, depleted oil and gas wells, deep saline aquifers, , or as
- naturally mineralised carbonate by injection into basalt reservoirs(see... basalt) Tests at the CarbFix project in Iceland indicate that most of the CO2 injected into basalt turned into carbonate minerals in less than two years, far shorter a time than the hundreds or thousands of years that scientists had once thought such a process would take. For carbon dioxide to transform into carbonate, the rocks into which the gas is injected need to have calcium-, magnesium- or iron-rich silicate minerals. A chemical reaction then occurs that converts the carbon dioxide and minerals into a chalky carbonate mineral.
- injection of CO2 into deep ocean - absorbed by sea water but acidifies the ocean so potentially major consequences to fundamental food cycles
Weighing up the costs...
Regardless of the technique, the outcomes for coal combustion are similar. The amount of emissions is reduced by 80-100%, while the cost of coal-fired electricity generation increases by at least the same amount.These costs come from building the capture plant, CO₂ transport pipelines and the sequestration plant.
More than double the amount of coal must be burned to make up for the energy cost of the CCS process itself.
The only way shifting to ultra-supercritical coal power could meet Australia’s 26-28% climate target is if carbon capture and storage (CCS) were applied.
Ultra-supercritical coal plants are expected to generate electricity at A$80 per megawatt-hour, according to the Australian Power Generation Technology Report.
This is 45% more expensive than the average wholesale cost of electricity for 2015-16.
If CCS is added, then the projected cost swells to A$155 per megawatt-hour, nearly three times last year’s wholesale cost of electricity.
"Clean" Coal electricity generation and Carbon Capture and Storage - CCS technologies sources
source:http://theconversation.com/is-clean-coal-power-the-answer-to-australias-emissions-targets-71785 , author Lynette Molyeaux, University of Queenslandhttps://theconversation.com/carbon-capture-and-storage-is-unlikely-to-save-coal-in-the-long-run-54182,
author Gary Ellem, University of Newcastle
http://reneweconomy.com.au/how-much-do-ultra-supercritical-coal-plants-really-reduce-air-pollution-70678/,
author Lauri Millyvirta
https://theconversation.com/building-a-future-for-carbon-capture-technology-6782,
author Paul Feron CSIRO
Nickel-base superalloys for ultra-supercritical coal-fired power plants: Fireside corrosion. Laboratory studies and power plant exposures,
authors G Stein-Brzozowska, DM Flórez, J Maier… - Fuel, 2013 - Elsevier
http://www.smithsonianmag.com/science-nature/iceland-carbon-capture-project-quickly-converts-carbon-dioxide-stone-180959365/
note: original texts edited and reduced by Earth Science Australia

