Salt Deposits
Salt Deposits
courtesy of The Salt Institute http://www.saltinstitute.org
What is salt?
Properties of Pure Sodium Chloride
Purity of rock salt
Toxic levels of sodium chloride
Salinity
Uses of Salt
Solar Salt Production
Solution Mining for Salt
Vacuum Pan Salt Refining
Rock Salt Mining
The oldest working salt mine in Europe
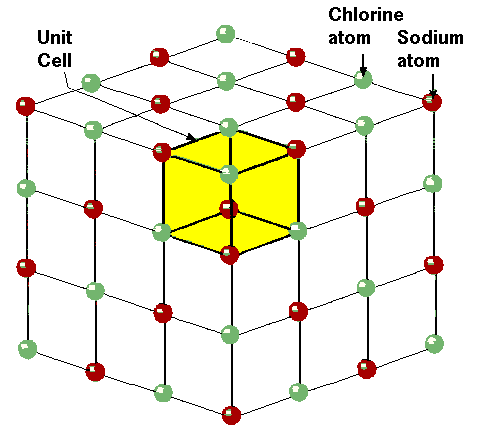
What is salt?
Sodium chloride or common salt is thechemical compound NaCl. It occursnaturally in many parts of the world as themineral halite and as mixed evaporites in saltlakes. Seawater contains an average of2.6% (by weight) NaCl, or 26 million metrictons cubic kilometer (120 million short tonsper cubic mile, an inexhaustible supply.Underground salt deposits are found in both sedimentary and domal deposits.
Properties of Pure Sodium Chloride
Molecular weight - NaCl58.4428Atomic weight - Na22.989768 (39.337%)
Atomic weight - Cl35.4527 (60.663%)
Eutectic composition23.31% NaCl
Freezing point of eutectic mixture-21.12° C (-6.016°F)
Crystal formisometric, cubic
Colorclear to white
Index of refraction1.5442
Density or specific gravity2.165 (135 lb/ft3)
Bulk density, approximate (dry, ASTM D 632gradation)1.154 (72 lb/ft3)
Angle of repose (dry, ASTM D 632 gradation)32°
Melting point800.8° C (1,473.4° F)
Boiling point1,413°C (2,575° F)
Hardness (Moh's Scale)2.5
Critical humidity at 20 °C, (68° F)75.3%
pH of aqueous solution neutral
Sodium chloride is sold in several different particle sizes (gradation) and forms, depending onthe intended end use. Fine granulesare typical of table salt and even finer popcorn salt. Kosher salt, pickling salt and ice cream saltare slightly coarser. Small compressed pellets are used in water softeners and large salt blocksare used as salt licks for livestock.
When viewed under strong magnification, all sodium chlorideis crystalline. Very large cubic crystals, of two, three or more inches in size, can be seen insome salt mines. They are transparent and cleave into perfect cubes when struck with a hard object.
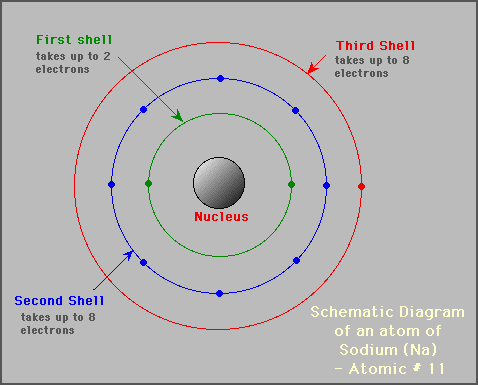
atomic structure of sodium atom.
It becomes an positive ion when it loses an electron
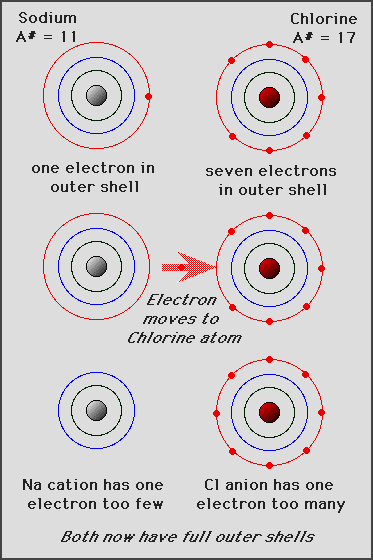
formation of sodium chloride (salt)
+positive charge on Na+(sodium ion) attracted to -negative charge on Cl-(chlorine ion)
Purity
of rock salt produced varies depending on the type of salt(evaporated, rock, solar) and on the source. Rock salt typically ranges between 95% and 99%NaCl, and mechanically evaporated salt and solar salt normally exceed 99% NaCl. Evaporatedsalt made with purified brine has the highest purity, in some cases 99.99% NaCl. Voluntarystandards, such as those developed by the American Society for Testing and Materials(ASTM), the American Water Works Association (AWWA) assure appropriate quality for theintended use. Mandatory specifications for food grade, drug/medical and analytical use includeFood Chemicals Codex, U.S. Pharmacopoeia, and Reagent Grade Chemicals.Common salt or sodium chloride is considered assafe for its intended use as a food additive. TheMerck Index refers to sodium chloride as "(n)ot generally considered poisonous." Manysubstances in everyday use can be toxic in high concentrations, even water. reports
Toxic levelsof sodium chloride are reported as:
Oral toxicityHuman; TDLo: 12,357 mg/kg/23 D-C
Mouse; LD50: 4,000 mg/kg
Rat; LD50: 3,000 mg/kg
Rabbit; LDLo: 8,000 mg/kg
Acute aquatic toxicity
Rana Breviceps (frog); No observed effect concentration (NOEC): 400 mg/L.
Daphnia pulex 48-hour LC50 or EC50: 1,470 mg/L
Daphnia magna (water flea); 48 hour EC50: 3,310 mg/L
Myriophyllum spicatum (water milfoil); Phytotoxicity (EC50 for growth): 5,962 mg/L
Pimephales promealas (fathead minnow); 69-hour LC50: 7,650 mg/L
Lepomis macrochirus (Bluegill) LC50 or EC50: 7,846 mg/L
Anguilla rostrata (American eel) 48-hour LC50 or EC 50: 13,085 mg/L
The chlorides of calcium, magnesium and potassium are generally more toxic tofresh water species than sodium chloride.
Salinity
Sodium and chloride occur naturally in soils and waters, and are added by residential,commercial and industrial activity. Aquatic organisms and vegetation, including crops androadside grasses, shrubs and trees, tolerate various concentrations of sodium and chloride.Thefollowing classification is used by the U.S. Department of Agriculture to indicate the degree ofhazard of saline soils to food crops. It is based on conductivity and salinity hazard.(Conductivity can be converted to approximate mg/L dissolved solids).
USDA Salinity hazard ratings:
Low: 70 - 175 mg/l
Medium: 176 - 525 mg/l
High: 526 - 1,575 mg/l
Very high: more than 1,575 mg/l
Factors that affect the degree of salinity hazard are: soil texture, soil permeability,drainage, quantity of water applied and the salt tolerance of the vegetation.
Uses of Salt
Every day, each of the earth's 5.5 billion inhabitants uses salt. Annual saltproduction has increased over the past century from 10 million tons to about 190million tons today. Nearly 100 nations have salt producing facilities ranging fromprimitive solar evaporation to advanced, multi-stage evaporation in salt refineries.Humans need salt to live. Prehistoric man obtained salt from the meat of huntedanimals. When man developed agriculture, salt was added to supplement thevegetable and cereal diet and the quest for salt became a primary motivation in history. In the mid-1800s, salt's value as an important raw material for thechemical industry was established when the Solvay process in Belgium convertedsalt to synthetic soda ash. Salt is, today, the largest mineral feedstock consumedby the world chemical industry.
Solar Salt Production
Solar salt is produced by the action of sun and wind on seawater or natural brine in large ponds.The water evaporates in successive ponds until the brine is fully concentrated and saltcrystallizes on the floor of the crystallizing ponds. Solar salt plants must be located in areas oflow rainfall and high evaporation rates, and where suitable low-cost is available.
Seawater contains about 3.5% (by weight) dissolved minerals. Sodium chloride is 77%
Solution Mining for Salt
Solution mining of salt or halite deposits is just like it sounds. Fresh and recycled water isinjected through a well (or wells) drilled into an underground salt bed, usually between 150 and1,500 meters (500 to 5000 feet) deep. Dissolution of the salt forms a void or cavern in the saltdeposit. Salt brine is withdrawn from the cavern and transported by pipeline to an onsiteevaporating plant to make dry salt, or to a chemical processing plant for chlor-alkali or otherchemical production. Solution mines located at the site of chemical plants are called captivebrine wells.Some salt solution mines consist of a single well with concentric casings extending into the saltcavern. Others consist of several adjacent wells extending into a single large cavern.
Brine iswithdrawn either through the outer concentric casing in a single well cavern, or through aseparate casing in a multiple well salt cavern. The size and shape of solution mined caverns canbe measured and controlled with well logging devices and operating techniques, thus minimizingthe potential for surface subsidence. After the end of use for salt or chemical production,solution-mined salt caverns are often used to store petroleum or other products.
Vacuum Pan Salt Refining
Table salt is typical of the fine, granulated-evaporated salt produced in vacuum pane vaporators.. Prior to mechanical evaporation, the brine may be treated to remove minerals that can cause scaling in the evaporators and adversely affect salt purity.Chemical treatment of the brine, followed by settling, reduces levels of dissolved calcium,magnesium and sulfate. Sulfuric acid treatment or chlorination may be used to remove hydrogen sulfide, and hydrochloric acid will neutralize brine used in diaphragm cell production of chlorineand caustic soda.
Brine purification has become increasingly important to produce high puritysalt for use in chlor-alkali production, particularly in Europe where dry salt is used extensively for this purpose.
Water is evaporated from purified brine using multiple-effect or vapor recompressione vaporators.
Multiple-effect systems typically contain three or four forcedcirculation evaporating vessels connected together inseries. Steam from boilers supplies the heat forevaporators and is fed from one evaporator to thenext to increase energy efficiency in the multiple effect system.
Vapor recompressionforced-circulation evaporators (pictured below)consist of a crystalliser, compressor and vapor scrubber. Feed brine enters the crystalliser vessel where salt is precipitated. Vapor is withdrawn, scrubbed and compressed for reuse in the heater.
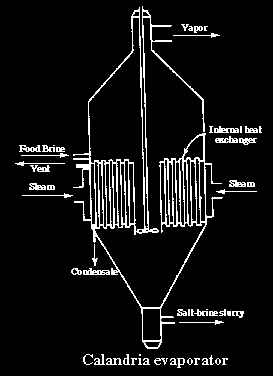
Recompression evaporators are more energy efficient than multiple effect evaporators,but require higher cost electrical power for energy input. The development of single stage compressors has significantly reduced costs.
Ultimately, weak brine from either process isrecycled to the solution mined cavern.Crystallized salt is produced as slurry which is dewatered first by centrifuging or vacuum drying andthen in kiln or fluidised-bed dryers where moisture content of the final product is reduced to 0.05% orless. During this century, salt producers have made significant advances in lowering energy consumption and in reducing salting and scaling in evaporators.
Rock Salt Mining
Salt occurs naturally in underground deposits, and occasionally in surface deposits in arid areas,as the mineral halite. Ancient salt deposits are widespread. There are ten major salt basins in the western hemisphere. A number of these salt deposits are mined for halite, commonly known asrock salt.
Salt deposits formed as horizontal salt beds in ancient oceans and were later buried deeply beneath sediments as mountains eroded. Later, some of these buried salt deposits were geologically deformed by tectonic forces within the earth. Salt domes (diapirs) are a major typeof salt structure resulting from tectonic deformation. Both bedded salt deposits and salt domes or diapirs are mined by drilling and blasting.
Bedded salt deposits are nearly horizontal, although some contain fault zones and other anomalies. Salt beds range in thickness from a few tens of feet to several thousands of feet. Saltdomes or diapirs formed as salt flowed plastically (because of pressure and heat) upward through overlying sediments. The result is a vertically elongated salt deposit of a mile or more in diameter and perhaps 15,000 to 20,000 feet in vertical length. The tops of some U.S. gulf coast salt domes are very near the surface.
Mining of both types of salt deposit is similar. The method of mining is called "room and pillar"because the salt is excavated by blasting and loading out a series of rectangular entries and cross cuts. Rectangular pillars in a checkerboard-like pattern, typically 35% to 50% of theoriginal salt, remain to support the mine roof. Rooms in bedded salt mines are 10 ft to 45 fthigh, while rooms in domal mines can exceed 100 ft in height
When a new mine is constructed, two shafts are excavated down to the salt deposit, usually between 500 feet to more than 2,000 feet deep. Shafts are about 20 ft in diameter and lined with concrete. After the shaft is sunk to the salt, rooms are mined in a planned pattern byundercutting, drilling and blasting. An undercutter cuts a horizontal slot or kerf along the floor ofthe advancing room to provide a second free face for blasting. A drilling rig drills a series ofholes into the face, and an ammonium nitrate-fuel oil mixture (ANFO) is pneumatically placed into the holes.
The room is then blasted, creating a "muck" pile of salt ready to be transported to an underground crushing and screen station. After loose pieces of salt are removed from the roof and pillars for safety reasons, the blasted salt is loaded into large trucks by front end loaders, or loaded by load-haul-dump units (LHDs) of lesser capacity that pick up the salt and haul it to the crusher.
After crushing, the salt is transported by conveyor belts to salt "pockets" at the shaft bottom for loading and hoisting to the surface. Two counter balanced salt skips of up to twenty short tons each are loaded automatically for the 1,000 ft/min trip. At some mines, the skip loading,hoisting, and surface dumping operations are managed completely by computers.
On the surface the salt is rescreened to remove fines, bagged, palletised and prepared for shipment to the salt customer. Most rock salt from underground mines in North America is transported in bulk by ship, barge, truck, or rail. Some is packaged in ten to 80 lb bags or compressed into salt blocks for animal nutrition and water softening.
Rock salt mines often have rich histories.
Wieliczka -site of the oldest working salt mine in Europe
In Cracow's proximate vicinity, 14 km south-east from the city center near Route 4 (E22), liesa well known country wide and abroad, city of Wieliczka with population of 20 thousand. The city is famous for having the oldest operational salt mine in Europe which has been working for over 700 years. This mine, a centuries old tourist attraction, cannot be compared to anything else in the world.The mine has been visited from its beginning by the famous and the mighty of this world.Kopernik, Goethe, Humboldt, Paderewski, Mendelejew, Baden Powell, Karol Wojtyla - who later became Pope John Paul II, ordinary people and crowned heads of state, all have visitedthis remarkable site.
Since 1978, the mine is on UNESCO's list of World Class Landmarks of Cultural and NaturalHeritage - where it was listed among the top twelve attractions in the world.
The tourist route open to sightseers is only a small fraction of the entire mine. The mine includes7.5 million square meters of post-excavation space on nine levels, each between 64 and 327meters.
The tourist route extends to level three only - to the depth of 135 meters.
During the two and half hour tour, visitors travel underground approximately 3.5 km., along passages totalling more then 320 km. During this time they will visit 30 of the more than 2148 chambers.
The most popular attractions are the enormous excavation chambers, the tallest being 36 meters high, and two others reaching 30 meters each. The tour includes a visit to three chapels, theoldest more than 300 years old and youngest a mere 100 years old. They are richly decoratedwith salt and wooden sculptures. The oldest salt sculptures date from the end of the 18thcentury.
The largest of the chapels - Blessed Kinga - is located 101 meters below the surface, it is over50 meters long, 15 meters wide, 12 meters high, and has the volume of 10,000 square meters.It is entirely made out of salt, richly furnished with sculptures, bas-reliefs, and large chandeliers made from salt crystals.
The three underground salt lakes are a real attraction, the deepest reaching seven meters. Signs of ancient mine works, wooden structures, machines and equipment hundreds of years old, may all be noticed throughout the tour. All this is located within the underground scenery,
remarkably rich in geological specimens which illustrate the complicated structure of this deposit. In many places, this beauty is most tangible.
An hour and a half into the tour, it is time to take a break in the "Warszawa" chamber which often serves as a sport, ball, theatre and even an opera hall!
The second part of the tour covers 10 chambers representing an underground exhibition of Cracow's Salt Mine Museum .
The collection includes antique tools, machinery and equipment,lighting equipment, as well as an exposition of surface archaeological excavations and interesting geological specimens.
The exhibition also includes painting portraying the work of miners in the old days, old models of buildings and mining structures, and a miniature model of Wieliczka 350 years ago as well as the cross section of the old mine. No description, no matter how detailed, will convey the charm of this mine and its underground attractions. An additional advantage of the mine is its underground microclimate which is especially beneficial for asthma sufferers. Sanatorium stays have even been organized here periodically.

