Hydrogen Energy in Australia – Green or Fossil Fuel ?
Hydrogen Energy in Australia – Green or Fossil Fuel ?
Hydrogen Overview
Storage/Transport
Extraction
from Methane CH4 (natural gas)
Extraction from Fresh Water
Other methods of
producing hydrogen
Hydrogen as an Export
Commodity
Potential Use Within
Australia
Hydrogen
Microgrid Project - Daintree Far North Queensland
What
are other countries doing with hydrogen?
Sources
see also Our Hydrogen Future and Australia as a Major Exporter of H2 Hydrogen- questions that need to be addressed
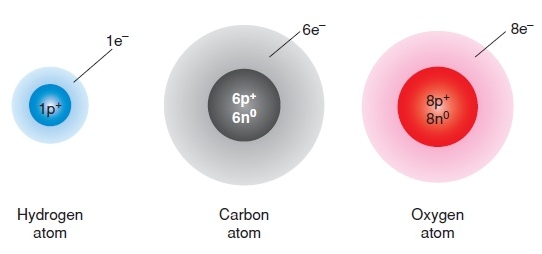
Hydrogen Overview
Hydrogen is the simplest element. Each atom of hydrogen has only one proton. Hydrogen is also the most abundant element in the universe.
Stars such as the sun consist mostly of hydrogen. The sun is essentially a giant ball of hydrogen and helium gases.
Hydrogen itself burns cleanly, leaving water as a by product.
Even though hydrogen itself is essentially non-polluting when burned (some nitrogen oxides, or NOx, may be formed), there is a carbon footprint associated with it.
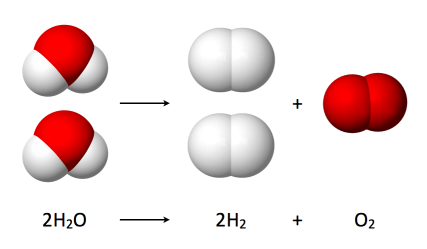
Hydrogen occurs naturally on earth only in compound form with other elements in liquids, gases, or solids namely:
- Water: Hydrogen combined with oxygen is water (H2O).
So if hydrogen is derived from water it is a renewable fuel with
virtually no nasty by products. Potentially it could be converted back
to easily transported liquid Electro-fuels
(E-fuel) through recombination with atmospheric carbon dioxide
though this product is the current subject of some debate
- Hydrocarbons (fossil fuels): Hydrogen combined with carbon
forms different compounds—or hydrocarbons—found in natural gas, coal,
and petroleum. The major source being Methane C2H4
If hydrogen is derived from hydrocarbons then it must be considered a
fossil fuel that also creates the greenhouse gases carbon dioxide or
carbon monoxide as by-products of the manufacturing process. CCS
carbon capture sequestration methods required to bury these
by-products require further substantial energy input.
Hydrogen gas is described by colours depending on how it is made
- White hydrogen - naturally occuring from rare underground sources
- Brown hydrogen - from conversion of coal
- Grey hydrogen – extracted from oil and natural gas producing greenhouse gases that contribute to global warming.
- Blue hydrogen – is as above but the carbon emissions in the production process are captured, sequestered, or repurposed so that they do not contribute to global warming.
- Turquoise hydrogen - experimental - from natural gas (methane) at high temperatures producing hydrogen and solid carbon
- Pink hydrogen- from nuclear energy
- Yellow hydrogen- electrolysis from a mix of renewable and non-renewable electricity sources.
- Green hydrogen – is produced from zero-emission renewable energy by electrolysis of fresh water

source:Geoscience Australia
Two characteristics are critical in understanding the usefulness of hydrogen as an energy source:
- Pro - Hydrogen has the highest energy content of any common fuel by weight (about three times more than petrol), but
- Con -Hydrogen has the lowest energy content by volume (about four
times less than petrol), so it is difficult to squeeze enough mass of
hydrogen into a reasonable volume
Hydrogen contributes about 30% of the energy content of methane. That means it takes about 3.3 m3 of hydrogen to deliver the same energy as 1 m3 of natural gas.
To produce hydrogen by electrolysis, 39.4 kWh of input power is required to produce one kg of hydrogen, if the electrolysis process is 100% efficient.
There are two primary ways to create hydrogen. In one of them, hydrogen is best thought of as a refined fossil fuel and is equivalent to natural gas. In the other, hydrogen is made by putting a lot of electricity into water and is best thought of as a store of energy like a battery.
When we talk about generating electricity, we typically talk about taking net positive amounts of energy from a source and creating electricity. For example, the net energy of generating electricity from natural gas (methane) is positive. We extract the methane, we do some limited processing of it such as add mercaptan to it to make it smell so we can detect leaks, we distribute it and then we burn it. The energy we receive from burning the methane is greater than the energy used to pump, process and distribute it.
Storage/Transport
The challenge with hydrogen as a transport fuel – and with storing and transporting hydrogen in general – is that it is an extremely light, low-density gas. If a fuel cell car were to use atmospheric pressure to store the 1kg of hydrogen needed to drive 100km, the fuel tank would have to be 11m3 in size. So today’s fuel cell cars use compressed hydrogen gas, squeezing about 5kg into a 700 bar carbon fibre reinforced tank.Tank Storage
But high pressure storage tanks are far from ideal
- they are heavy, awkwardly shaped, expensive fuel container
- it takes a lot of energy to compress the gas,
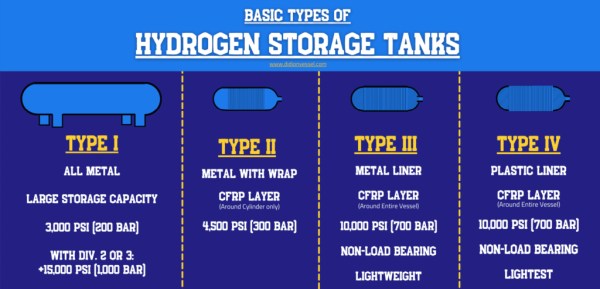
TYPE I Hydrogen Storage Vessels: - All Metal Design
This type of Hydrogen Tank is the least expensive to manufacture, is fabricated from an all-metal cylinder, and can be built to huge sizes. These are the heaviest Hydrogen Storage Tanks and usually operate at lower pressures than the other types of vessels listed. The mass of the metal required to contain the pressure in a Type I Tank usually only allows for 1% to 2% hydrogen storage compared to the cylinder mass. So, the mass of hydrogen stored to the mass of cylinder ratio is very low.The Type I Hydrogen Tanks also include ultra cold (cryogenic) storage of hydrogen as a liquid but these must deal with Boil-off gas (BOG), a unique challenge to cryogenic liquids. To remain in its liquid phase, hydrogen must maintain cryogenic temperatures. If temperatures rise in the vessel, the hydrogen will boil, changing from a compact liquid into an expanding gas. If left unchecked, this will push your vessel beyond its design pressure.
Type II: All Metal With Carbon Fibre
These cylinders are all metal and are hoop wrapped (wrapped around the straight cylinder portion) with composite material. T he stored hydrogen mass to cylinder mass ratio is very low. These vessels are also heavy and thick-walled. Standard tanks usually have a maximum pressure of 4,500 psi (300 Bars).Type III: Composite With Metallic Lining
This Hydrogen Storage tank has a much more efficient capacity: up to four times that of standard Type I vessels. This means smaller and lighter cylinders can be used to store the same amount of hydrogen. These vessels are fully wrapped composite cylinders and have a metallic lining. They are non-load bearing. Standard tanks usually have a maximum pressure of 10,000 psi (700 Bars).Type IV: Composite With Non-Metallic Lining
This style has the same efficient storage capacity as the Type III’s. They are fully wrapped composite cylinders with non-metallic liners, usually consisting of some sort of polymer (like high-density polyethylene). Type IV Vessels are non-load bearing.Standard tanks typically have a maximum pressure of 10,000 psi (700 Bars). Type III & IV tanks are the priciest of all the Hydrogen Storage Tanks due to the current expense of carbon fiber composite material. Although, many believe that the cost of carbon fiber will lower in the future.
To transport bulk quantities of hydrogen, however – whether to supply refuelling stations, or to ship renewable hydrogen around the world – pressuring the gas is not going to work due to what is called the “Chilling Effect” namely...
The dihydrogen molecule, with its two protons, can form two possible quantum spin isomers. In para hydrogen, the more stable form, the spin on the two protons is opposite; in ortho hydrogen, the spin is aligned. At room temperature, about 75% of dihydrogen is in the higher energy ortho state, but the equilibrium shifts as the temperature is lowered, until at –253°C, it is almost 100% para at equilibrium. As the liquified gas heats para reverts to ortho and energy is released causing the liquid to boil.
Currently, liquefying hydrogen takes 12kWh of power per kilo of hydrogen, equivalent to about 25% of the energy that hydrogen would release in a fuel cell.
In short tanks only holding hydrogen gas are not proactical being bulky, heavy and expensive.
Using an Adsorbent in a Tank
A tank is filled with special material that will absorb hydrogen or that hydrogen will stick to.Researchers seek this ideal tank, for various parameters not discussed here, the ideal tank would have a maximum pressure of 100 bar and with hydrogen adsorption energy of 15–20kJ/mol.
At that pressure, light-weight, inexpensive, conformable pressure vessels are possible and the energy penalty for compressing the hydrogen would also be slashed.
The problem for physical adsorption it that hydrogen does not like to stick to things.
The two main classes of solid-state hydrogen storage materials sit either side of that ideal tank.
- Metal hydrides, and related materials that take up hydrogen
via chemical bond formation, bind hydrogen too strongly and have to be
heated to drive the hydrogen off. Metal hydrides are metals which have
been bonded to hydrogen to form a new compound.The most common
examples of metal hydrides include aluminum, boron, lithium
borohydride and various salts. For example, aluminum hydrides include
sodium aluminum hydride.
- Metal-organic frameworks (MOFs) are porous absorbents but
currently bind hydrogen too weakly to hold enough of it. MOFs are a
class of organic-inorganic hybrid crystalline materials consisting of
metallic moieties that are linked by strong coordination bonds to
organic ligands. They exhibit a great structural diversity and possess
low weight, exceptionally high surface areas, large free volumes, and
tunable pore sizes and functionalities, making them extremely
attractive for a variety of applications such as hydrogen storage.
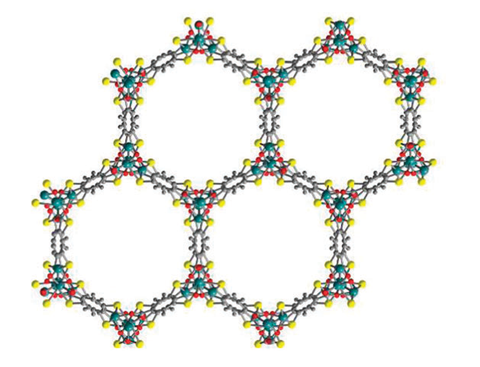
metal hydride - hydrogen in yellow
Generally hydrogen will be released from an absorption tank by heating the contents to release the hydrogen.
The tank can then be re-filled with new hydrogen.
Hydrogen Embrittlement occurs when metals become brittle as a result of the introduction and diffusion of hydrogen into the material. Hydrogen has handling and safety issues that methane does not. Hydrogen can cause embrittlement of metals, and deterioration of plastic and rubber seals.
The degree of embrittlement is influenced both by the amount of hydrogen absorbed and the microstructure of the material. Hydrogen is normally only able to enter metals in the form of atoms or hydrogen ions.
Thus, gaseous hydrogen is not absorbed by metals at ambient temperatures, as it is in molecular form, in which pairs of atoms are tightly bound together.
Hydrogen ions are also produced by reactions associated with processes such as corrosion, electroplating and cathodic protection. Consequently, there is ample opportunity for the entry of hydrogen into metallic components.
Extraction from Methane CH4 (natural gas)
Two methods are discussed Steam, Methane Reforming and Partial OxidationOver 95% of the world’s hydrogen is produced using the steam methane reforming process (SMR). In this reaction, natural gas is reacted with steam at an elevated temperature to produce carbon monoxide and hydrogen. A subsequent reaction — the water gas shift reaction — then reacts additional steam with the carbon monoxide to produce additional hydrogen and carbon dioxide.
1.Steam-Methane Reforming
Most hydrogen produced today is made via steam-methane reforming, a mature production process in which high-temperature steam (700°C–1,000°C) is used to produce hydrogen from a methane source, such as natural gas. In steam-methane reforming, methane reacts with steam under 3–25 bar pressure (1 bar = 14.5 psi) in the presence of a catalyst to produce hydrogen, carbon monoxide, and a relatively small amount of carbon dioxide. Steam reforming is endothermic—that is, heat must be supplied to the process for the reaction to proceed.Subsequently, in what is called the "water-gas shift reaction," the carbon monoxide and steam are reacted using a catalyst to produce carbon dioxide and more hydrogen. In a final process step called "pressure-swing adsorption," carbon dioxide and other impurities are removed from the gas stream, leaving essentially pure hydrogen. Steam reforming can also be used to produce hydrogen from other fuels, such as ethanol, propane, or even gasoline.
Steam-methane reforming reaction
CH4 + H2O (+ heat) → CO + 3H2
Water-gas shift reaction
CO + H2O → CO2 + H2 (+ small amount of heat)
The Carbon Footprint of Steam Methane Reforming The carbon footprint of hydrogen production via SMR can be broken down into two parts.
First, as indicated by the SMR and WGS reactions, 100% of the carbon in the incoming methane is ultimately converted to CO2. In the process of producing one molecule of CO2, four molecules of hydrogen (H2) are produced, with the steam contributing the additional hydrogen.
9.3 kilograms (kg) of CO2produced per kg of hydrogen production
For hydrogen created by steam reformation of methane. We extract the methane, use a bunch of energy in the steam reformation process where the carbon is removed and turns into CO2 other elements such as nitrogen and sulfides are also removed and typically turn into other air pollution, distribute the remaining hydrogen and then use it in a fuel cell or burn it. That’s highly similar to methane except that instead of burning both the hydrogen and the carbon in the methane and extracting the energy, we waste the energy in the carbon and still get CO2. But we are still getting a net positive energy extraction from the hydrogen at the end of the process, just less than from burning the methane directly.
Summary - Hydrogen from steam reformation of methane
Pros
- Net positive source of energy.
- No negative emissions at end point of use.
- Less expensive than hydrogen from electrolysis.
- Processing emits just as much CO2 as burning the methane directly, so it contributes to global warming.
- It’s energy inefficient compared to burning the methane in a combined cycle gas generator to get much more of the energy.
- Nitrous and sulfur oxides are emitted by processing and create air pollution.
- Extracting methane is shown to have significant leakage of methane to the atmosphere, and methane is a much more potent greenhouse gas than CO2.
- There are local impacts of fracking such as minor earthquakes and in some cases ground water pollution to consider.
2. Partial Oxidation
In partial oxidation, the methane and other hydrocarbons in natural gas react with a limited amount of oxygen (typically from air) that is not enough to completely oxidize the hydrocarbons to carbon dioxide and water. With less than the stoichiometric amount of oxygen available, the reaction products contain primarily hydrogen and carbon monoxide (and nitrogen, if the reaction is carried out with air rather than pure oxygen), and a relatively small amount of carbon dioxide and other compounds. Subsequently, in a water-gas shift reaction, the carbon monoxide reacts with water to form carbon dioxide and more hydrogen.Partial oxidation is an exothermic process—it gives off heat. The process is, typically, much faster than steam reforming and requires a smaller reactor vessel. As can be seen in chemical reactions of partial oxidation, this process initially produces less hydrogen per unit of the input fuel than is obtained by steam reforming of the same fuel.
Partial oxidation of methane reaction
CH4 + ½O2 → CO + 2H2(+ heat)
Water-gas shift reaction
CO + H2O → CO2 + H2 (+ small amount of heat)
Extraction from fresh water
Australia is a very dry continent and fresh water is and will remain at a premium however extraction of hydrogen from water using surplus renewable electricity from wind and solar sources could assist in generating supplementary electricity during periods of peak demand or for bulk transport.Electrolysis of water to create hydrogen, is a pure energy store. We take electricity generated by some other means, typically with some mix of fossil fuel generation in it, we use that electricity in the lossy electrolysis process, we capture the resulting hydrogen, we distribute it and then we use it in a fuel cell or burn it to generate electricity. That’s much more similar to a battery.
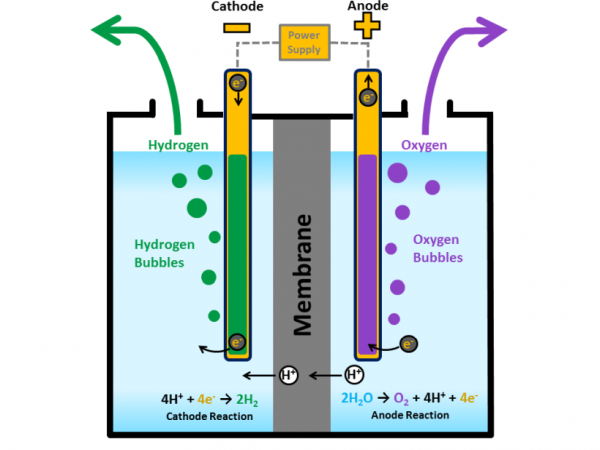
Electrolysis is the process of using electricity to split water into hydrogen and oxygen. This reaction takes place in a unit called an electrolyser.
PEM electroliser
Types of Electrolisers
Like fuel cells, electrolysers consist of an anode and a cathode separated by an electrolyte.Different electrolysers function in slightly different ways, mainly due to the different type of electrolyte material involved.
Polymer Electrolyte Membrane PEM Electrolysers
In a polymer electrolyte membrane (PEM) electrolyser, the electrolyte is a solid specialty plastic material.
- Water reacts at the anode to form oxygen and positively charged
hydrogen ions (protons).
- The electrons flow through an external circuit and the hydrogen
ions selectively move across the PEM to the cathode.
- At the cathode, hydrogen ions combine with electrons from the
external circuit to form hydrogen gas. Anode Reaction: 2H2O
→ O2 + 4H+ + 4e- Cathode Reaction: 4H+ + 4e- → 2H2
Alkaline Electrolysers
Alkaline electrolysers operate via transport of hydroxide ions (OH-) through the electrolyte from the cathode to the anode with hydrogen being generated on the cathode side. Electrolysers using a liquid alkaline solution of sodium or potassium hydroxide as the electrolyte have been commercially available for many years. Newer approaches using solid alkaline exchange membranes as the electrolyte are showing promise on the lab scale.
Solid Oxide Electrolysers
Solid oxide electrolysers, which use a solid ceramic material as the electrolyte that selectively conducts negatively charged oxygen ions (O2-) at elevated temperatures, generate hydrogen in a slightly different way.
- Water at the cathode combines with electrons from the external
circuit to form hydrogen gas and negatively charged oxygen ions.
- The oxygen ions pass through the solid ceramic membrane and react
at the anode to form oxygen gas and generate electrons for the
external circuit.
When electricity is used to produce hydrogen, thermodynamics dictate that you will always produce less energy than you consume. In other words, the energy input in electricity will be greater than the energy output of hydrogen. Nevertheless, if a cheap source of electricity is available — such as excess grid electricity at certain times of the day — it may be economical to produce hydrogen in this way. In Australia water usage in electrolysis should be a “closed circuit” with by-product water being returned to the electrolyser for multiple re-use.
Summary - Hydrogen from electrolysis
Pros
- If the electricity for electrolysis is sourced from renewables with low CO2, then the net energy cycle is very low carbon.
- No negative emissions at end point of use.
- Electrolysis is about 70% efficient, meaning about 30% of the energy in the electricity is wasted. This is much less efficient than batteries.
- If the electricity for electrolysis is sourced from fossil fuels, then the net energy cycle is higher carbon.
- Fuel cells are only 40% to 60% efficient and waste heat is generated. If the waste heat is used as well, overall efficiency at point of generation can be greater, but the theoretical maximum is 85%. At minimum, 15% of stored electricity is thrown away. In reality, no automotive fuel cell captures the waste heat, so 40% to 60% of the stored electricity is thrown away.
- If the hydrogen is burned in a Carnot or steam cycle, then efficiency is even lower than via a fuel cell, closer to gasoline where efficiency is in the range of 20%.
Other methods of producing hydrogen
Solar-Driven Processes
Solar-driven processes use light as the agent for hydrogen production.
There are a few solar-driven processes, including photobiological, photoelectrochemical, and solar thermochemical.
Photobiological processes use the natural photosynthetic activity of bacteria and green algae to produce hydrogen.
Photoelectrochemical processes use specialized semiconductors to separate water into hydrogen and oxygen.
Solar thermochemical hydrogen production uses concentrated solar power to drive water splitting reactions often along with other species such as metal oxides.
Biological Processes
Biological processes use microbes such as bacteria and microalgae and can produce hydrogen through biological reactions.
In microbial biomass conversion, the microbes break down organic matter like biomass or wastewater to produce hydrogen, while in photobiological processes the microbes use sunlight as the energy source.
Hydrogen as an Export Commodity
At time of writing the Federal government has allocated $539 million on “hydrogen” production and CCS “carbon capture sequestration” at five “hubs”.In Australia, ammonia NH4 is being eyed favourably as a way to export hydrogen.
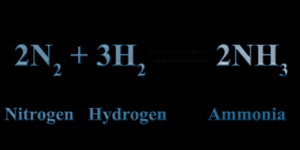
The Haber Process is used to convert hydrogen to Ammonia at about 400 to 500 degrees Celsius and 100 bar.
Presently ammonia is produced from fossil fuel sources. . The process of converting hydrogen to ammonia at source and then converting the ammonia back to hydrogen by the purchaser involves the release of waste products including nitrous oxides.
Haber Process for making Ammonia
Australia is home to the world’s largest carbon capture and storage facility at Chevron’s Gorgon natural gas project on Barrow Island off its northwest coast.
The project has stored more than 4 million metric tons (4.4 million U.S. tons) of carbon emissions since it started operating in 2019. CCS Carbon Capture Sequestration involves mechnically compressing CO2 carbon dioxide to an ultra cold supercritical liquid s-CO2. The s-CO2 is injected via drill holes deep into geological strata where, in theory the pressure of the overlying rock keep the s-CO2 in a near supercritcal state. The host rock strata chosen must be overlain by an "impermeable" cap-rock layer meant to prevent any s-CO2 from migrating upward to the surface.
Built by Chevron in 2016 at a likely cost of $88 billion, the facility produces 25 million metric tons of natural gas and up to 10 million metric tons of carbon dioxide each year.
That’s because the Gorgon gas field contains a mix of about 60% natural gas and 40% carbon dioxide (which exists in many gas fields around the world).
Chevron has to separate the carbon dioxide from the mixture before it can liquefy natural gas for transport.from the mixture and sequester it underground.
New money would be spent on accelerating development of a new carbon capture hub and technologies and the conversion of natural methane gas to exportable ammonia.
The company said that, after initial tests are complete, it will bury as much as 4 million metric tons of carbon dioxide annually—cutting the project’s carbon footprint by about 40%.
Most CCS facilities inject the captured carbon dioxide into oil fields, where CO2 dissolves the oil and actually increases the volumes that can be recovered.
In Norway, Equinor (formerly Statoil) buries carbon dioxide in saline aquifers, because releasing the gas would require paying a steep carbon price.
That’s what makes the Gorgon project different.
The carbon dioxide buried is neither used for enhanced oil recovery nor is it buried to avoid a carbon price.
It’s being buried simply because the Australian government asked Chevron to do so.
Once working at full scale, it will be the world’s largest CCS facility that simply buries the greenhouse gas in underground reservoirs.
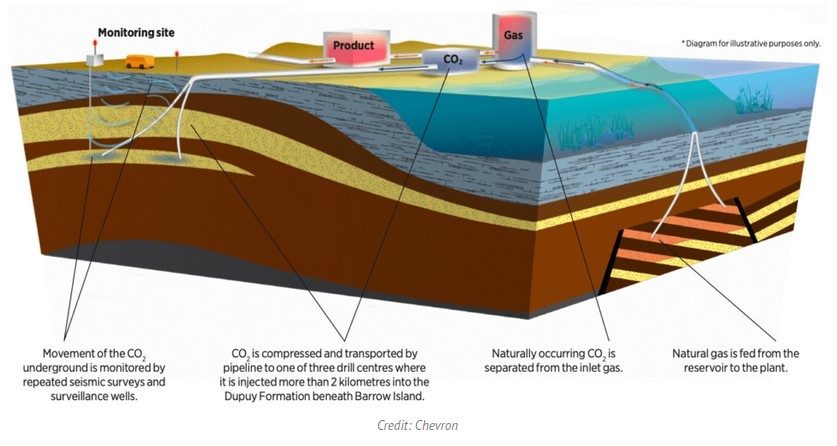
The Carbon Dioxide (CO2 Injection Project involves the design, construction and operation of facilities to inject and store reservoir CO2 into a deep reservoir unit, known as the Dupuy Formation, a deep saline formation, more than two kilometres beneath Barrow Island. The Dupuy Formation is Late-Jurassic aged and consists of sandstones and siltstones, with overall thickness of between 200m and 500m.
Permanently storing CO2 in subsurface geologic reservoirs has been proposed as a means to curb anthropogenic CO 2 emissions into the atmosphere. Storage of CO 2 in geologic reservoirs will occur in the forms of structural or stratigraphic trapping, residual or capillary trapping, solubility trapping, and mineral trapping.
Among these, residual or capillary trapping has emerged as one of the most significant mechanisms for the long‐term geological storage of CO 2
The reservoir CO2 will be separated at the liquefied natural gas (LNG) plant site and transported by pipeline to one of three drill centres. To minimise the environmental footprint on the island, nine injection wells have been directionally drilled from the three drill centres. Once the CO2 is injected, it will migrate through the Dupuy Formation until it becomes trapped
The top of the Dupuy Formation reservoir is located approximately 2300 m below Barrow Island and is overlain by a thick shale cap-rock seal. The shale is intended to keep most of the entrapped carbon dioxide from leaking to the surface. The pressure in the reservoir will cause the injected CO 2 to behave as a supercritical fluid with behavio ur of both a liquid and a gas.
The reservoir CO 2 will become trapped in the reservoir through a combination of residual saturation trapping and by dissolution into the waters in the formation.
During geologic CO2 sequestration, the liquid carbon dioxide (referred to a supercritical) s-CO2 is injected into the sandstone and siltstone.
Salty water (native brine) is already present in the tiny pore spaces (capillaries) between the rock grains. The s-CO2 moves upward in the salty water some dissolves in the water and some gets trapped in the pore spaces as bubbles or ganglia.
Some considerations along the way….
- no geological structure is without flaws so some leakage may be
expected.
- due to delays in CCS the project accounted for about half the
greenhouse gas increase Australia saw in 2018.
- only 40% of CO2 produced is currently successfully
captured and injected
- core‐scale experiments and pore‐network flow models suggest that
this capillary trapping in saline aquifers ranges from 10% to 90% of
the total injected volume of CO2. So not all injected CO2
remains in-situ
- at the producer end conversion of hydrogen to ammonia releases
greenhouse gases
- at the buyer end conversion of ammonia back into hydrogen
releases greenhouse gasses
Untested Experimental Zero CO2 process that converts methane into hydrogen
West Virginia University (WVU) has developed a process that converts methane—the primary component of natural gas—into hydrogen while emitting zero CO 2.
Pros
- The process creates carbon solids for manufacturing applications,
valuable for early producers The solid carbon nanocrystals that
accumulate on the catalyst can washed and separated for commercial
use in carbon fibres
- The thermocatalytic decomposition (TCD) method process uses a
novel nickel-based bimetallic catalyst to produce hydrogen at 600
degrees Celsius
- the metallic precursors are re-synthesised and recycled back into the reactor.
- the closed-loop cycle allows for continuous catalyst replacement while emitting zero carbon dioxide emissions.
- the downside is that with commercial levels of production the world market would be flooded with high quality carbon suitable for carbon fibre but the supply would greatly exceed demand and carbon prices
- high temperatures required
- the process has not been tested at scale
Ammonia to Hydrogen conversion using renewable electricity
Northwestern University has developed an experimental electrochemical cell with a proton-conducting membrane and integrated it with an ammonia-splitting catalyst.
The process functions at much lower temperatures than traditional methods (250 degrees Celsius as opposed to 500 to 600 degrees Celsius)
The ammonia first encounters the catalyst that splits it into nitrogen and hydrogen.
That hydrogen gets immediately converted into protons, which are then electrically driven across the proton-conducting membrane in our electrochemical cell. Using Le Chatelier's principle by continually pulling off the hydrogen, the reaction is driven further than it would otherwise. Removing one of the products of the ammonia-splitting reaction—namely the hydrogen—pushes the reaction forward, beyond what the ammonia-splitting catalyst can do alone.
Potential Use Within Australia
“Hydrogen” cannot always be associated with “Green”So it would seem that our export hydrogen would be produced from natural gas rather than via solar using water.
Without 100% CCS this process contributes to carbon dioxide pollution of our atmosphere – the hydrogen produced is a “refined fossil fuel” and has a negative impact in terms of stabilising our atmosphere and climate.
At the time of writing $83 million dollars of government money in New South Wales is being allocated to the construction of a natural gas/ hydrogen electricity co-generation facility initially burning natural gas but able to burn 95% natural gas and 5% hydrogen in a couple of years’ time. The source of this “offer to buy” future hydrogen gas (water or fossil fuel) component currently does not exist and the manner in which the hydrogen will be produced and delivered has not been specified. It is possible to mix hydrogen and natural gas in the fuel stream, with a reduction in CO2 output, but because of the difference in energy content, to achieve a 50% reduction in CO2 requires about 75% H2 by volume.
One concludes that the NSW project is simply natural gas generator of electricity with the word “hydrogen” in there as a distraction of the reality another fossil fuel facility.
Hydrogen Fuel as part of a mix of energy sources
Prioritising to applications like aviation and steel productions
Electro-fuels (E-fuels) – Hydrogen from Electrolysis + CO2 from the atmosphere
So-called green hydrogen is produced through a process called electrolysis. To crack the stable H2O water molecules into Hydrogen and Oxygen, a lot of electricity is needed. The hydrogen can then be used to synthesise hydrocarbon fuels by adding carbon from CO2. The resulting electro-fuels or e-fuels are easier to store and transport than electricity or pure hydrogen.
Driving a car with hydrogen-based fuels needs five times more energy than a battery-electric car
Summary of Practicality for Australia
Australia is blessed with massive solar and wind energy potential. We also have quality reserves of lithium, magnesium, high quality silica sand and rare metals. Government value-adding investment in building and exporting quality solar, wind, semiconductor and lithium battery devices might be money better spent.
Hydrogen should only have a few specific roles in our energy mix. Only hydrogen production from closed circuit electrolysis should be considered. Our limited fresh water resources limits the range of hydrogen use to...
- contributing to peak electricity demand
- long-distance aviation
- bulk transport,such as trains, where fuel volume is a minor
factor
- alternative feedstocks in chemical production,
- clean high quality steel/ alloy production with focus on value
added alloys
- some industrial processes such as micro-chip manufacturing
CCS - Carbon capture sequestration has been studied for several decades. Results are not remotely close to the required 100%. Larger scale attempts fall back to a ”hide and forget” methodology.
The consequences of burying vast amounts of supercritical CO2 in geological strata for geological periods of time seems as environmentally irresponsible as the dumping of barrels of nuclear waste in to the deep ocean in the 1960's. Putting millions of tonnes if carbon dioxide into geologcal strata and expecting all of it to remain intact over geological periods of time borders on the absurd. Carbon dioxide will leak, the crust of the earth is dynamic and we risk acidifying oceans and groundwater, suffocating livestock and communities.
Hydrogen Microgrid Project - Daintree Far North Queensland
The World Heritage-Listed Daintree Rainforest region in northern Queensland has no or limited mains electricity supply. Residents of the area use diesel generators or solar power and battery storage, or a combination of both. Solar panels are not viable for all residents due to shading issues and, in the wet season, the lack of sunshine means diesel generators must be used. It is estimated that the Daintree area uses around four million litres of diesel per annum to generate power. The Federal Government is granting substantial funding to Daintree Renewable Energy Pty Ltd in support of a feasibility study to investigate the technical and economic viability of a proposed solar-based (with hydrogen storage) microgrid for the Daintree region. Work commenced on the study in late 2019 and is expected to be completed around the middle of 2020. The proposed microgrid project under evaluation would convert excess energy generated from existing and new solar panels into hydrogen via electrolysis. The hydrogen produced would be stored to be used to generate electricity during unfavourable conditions (thereby reducing reliance on diesel generation).
Following the May 2021 Budget, the local member stated on ABC radio, that the project would include solar panels, hydrogen production (via electrolysis?) AND battery storage. Fuels Cells are not mentioned.The area is one of extreme rainfall amounting to several metres per year. The sun can be behind heavy cloud for weeks at a time during the "Wet season". So why build a micro-grid that is solely dependent on sunlight to gererate both electricity and produce hydrogen as fuel to cover peak demand and provide electricity during the very long periods of thick cloud cover?
There are numerous major and minor permanent streams, all with sufficient head for a mix of high head / low water consumption (ie 50m and 2L/sec) and low head / high water consumption (ie 2m and 110L/sec) readily available micro-hydro facilities both of which would require minor infastructure and have minimal environmental impact.
The premise for the project seem counter-intuitive. When the sun isn't shining, it is raining and the streams are running well. When it is sunny the solar panels supply power and surplus can be put into batteries or flywheels or pumped hydro or compressed air- so why use hydrogen which would put additional demand on the solar panels and need sunny days? Is this project being set up to fail?
For this project based on publically availble information (May 2021)..When the sun is shining the solar panels must produce:
- demand electricity plus
- charge the batteries plus
- electrolise hydrogen
- in a RAINFOREST! with mean annual sunshine for 2019 only 20.0 MJ/m2 and high daily variations (courtesy bom.gov.au)
Sunverge was engaged by ARENA to produce "Powering Daintree A study of supply options for the Australian Renewable Energy Agency" here is some of their thinking...
- there are various different technologies currently available from fast response battery systems to fuel cells (power to gas/H2 and back to power)
- in the situation of the Daintree, baseload needs to be supported. Baseload support via batteries is extremely expensive and so the fuel cell options have been explored
- long-term energy storage is also problematic for batteries due to self/internal discharge, while this is very small large long-term storage losses due to this phenomenonis problematic.
- the impact of seasonality on utilisation factors for battery storage makes the investment in long term storage options too expensive when compared to other solutions.
- In Northern Europe due to the high uptake of seasonally-sensitive renewables(especially solar)grid supporting systems are being developed.
- During the market sounding exercise Sunverge approached a company calledITMwho currently have a number of published, established Power to Gas (Fuel Cell) demonstration projects with major European power companies. theimpact
- using H2 storage was seen as an alternative to batteries
- Due to the low cost to physically store H2 per kWh this allows for a simplistic protection approach in that the highest priority after safety is grid stability and so any deviation outside the allowable bands include rates of change in frequency will trigger the fuel cell to support the network (next level net load following PV excess)
The use of fast electrolysis and fuel cells does not seem to be mentioned in the current plan?
What are other countries doing with hydrogen?
- Australia is positioning themselves to be at the forefront of the much needed hydrogen market,heavily investing government money primarily blue and grey hydrogen refined from natural gas (methane) necessitating injecting underground geological structures to sequester some, but not all, carbon dioxide . Simultaneously AUD$240 million to develop the world’s largest ‘green’ ammonia plant called H2U. Situated in Southern Australia’s city of Whyalla, the plant will use a combination of wind and solar power to generate power to the 75MW electrolyser, which in turn will provide sufficient renewable green hydrogen gas via electrolysis to create 40,000 tonnes of ammonia each year. In periods of low renewable output, there will be two 16MW ‘open cycle gas turbines’ operating entirely on hydrogen produced at the site. Ammonia has the additional benefit of as being an alternative fuel source for large-scale power stations as a substitute for hydrocarbon-based fuels. While hydrogen gas is best utilised in the distribution to the end user, ammonia pellets made with hydrogen can used as feedstock.
- Saudi Arabia - the worlds largest oil producer is going for renewable green hydrogen via electrolysis. The intention being to remain as the central supplier of energy to the world. Saudi’s Public Investment Fund has partnered with Air Products to develop the $5 billion ‘mega-scale’ facility, the NEOM Project,the first ‘green’ hydrogen plant of its kind in the world. The initial phase of production will be powered by 4GW of an undeclared mix of wind and solar PV technology, and will produce 650 tonnes of green hydrogen per day.To facilitate the export of hydrogen, Neom will contain an ammonia production plant, one of the few processes capable of safe transport of hydrogen and with potential as a hydrogen-based fuel. This plant is necessary toward creating an export industry that intends to ship 1.2 million tonnes of ammonia each year.
- Japan - As the world’s fourth largest consumer of oil and gas products and one of the world's most nuclear based economies, Japan is going for renewable green hydrogen via electrolysis While Japan will be among the biggest importers of clean hydrogen in the coming years, the need to develop an internal supply is just as important.The result is the Fukushima Hydrogen Energy Research Field or FH2R, a joint venture by the New Energy and Industrial Technology Development Organisation (NEDO) – Japan’s premier public research institution, regional utility Tohoku Electric Power Company, Toshiba ESS and Iwatani Corporation.FH2R is a 10MW-class hydrogen production unit, that utilizes 20MW of solar PV generation alongside input from the grid to conduct the electrolysis of water. Using only renewable-powered electrolysis, FH2R can produce 1,200 Nm³/Hour or up to 900 tonnes of hydrogen a year. While this dwarfs in comparison to Neom’s expected output, the research facility reaches these figures with a relatively small 180,000m² solar PV field.
- Europe has been leading the hydrogen revolution for a number of years already as the region looks to replace the deep seasonal dependency on natural gas with hydrogen gas.They will be producing renewable green hydrogen gas via electrolysis One of the most ambitious projects is a joint venture between Gasunie, Equinor and Shell together with the local authorities of Groningen – called NortH2. NortH2 will be the largest offshore wind-powered hydrogen facility initially capable of producing 4GW of green hydrogen by 2030 and rising to 10GW by 2040, or totaling around 1 million metric tonnes each year. This equates to supplying the energy demands of 12.5 million Dutch households at their current levels. With the EU’s goal of developing 40GW of hydrogen electrolysers by 2040, this project will fulfill 10% of the continental supply! Beyond the benefit of developing ‘green’ hydrogen, there is the benefit of repurposing Gasunie’s existing natural gas infrastructure for the transmission and distribution of hydrogen gas. The potential in this area is massive considering the extensive gas infrastructure that exists throughout Europe. The sufficient capacity to store and transport hydrogen and the development of industrial clusters can ensure repurposing upgrades and costs are minimized by utilising existing infrastructure.While these ‘green’ hydrogen projects are the ideal end-goal, it cannot be overlooked that over 95% of hydrogen currently produced is done so as ‘blue’ or ‘grey’ hydrogen. The refining sector has assumed a monumental undertaking to support and grow the market and technologies required to kick start a functional hydrogen sector Central to how society embraces hydrogen will be defined by how we can utilize the gas (or it’s byproducts such as ammonia) beyond industrial or manufacturing capacity. And while hydrogen fuel cells for cars leave significant challenges still to be overcome, hydrogen-powered public transport has become an increasingly viable and attractive alternative
- Russia - there has been an impetus to search for ways to mitigate the greenhouse gas emissions as the future of fossil fuels remains in doubt. it is the development of low-carbon processes is needed to inhibit the advancement of climate change Building the first experimental turbine in the world to run entirely off hydrogen and designing gas turbines that can run on natural gas supplemented with 10% hydrogen
Sources
https://www.eia.gov/energyexplained/hydrogen/ https://qz.com/1684903/chevron-starts-gorgon-gas-project-that-buries-co2-underground/ by Akshat Rathi
https://www.theguardian.com/environment/2018/nov/14/half-of-australias-emissions-increase-linked-to-was-gorgon-lng-plant
https://www.intelligentliving.co/chevron-100m-fine/
https://www.energy.gov/eere/fuelcells/hydrogen-production-natural-gas-reforming
https://www.chemistryworld.com/features/hydrogen-storage-gets-real/3010794.article by Mark Allendorf https://phys.org/news/2020-11-technique-seamlessly-ammonia-green-hydrogen.html
https://abcnews.go.com/International/wireStory/australia-plans-spend-417m-hydrogen-carbon-capture-77210038
https://www.forbes.com/sites/quora/2018/05/08/what-are-the-pros-and-cons-of-using-hydrogen-to-generate-electricity/?sh=1f055eed34f5 by Michael Barnard, Low-carbon Innovation Strategist https://www.energy.gov/eere/fuelcells/hydrogen-production-electrolysis https://www.energy.gov/eere/fuelcells/hydrogen-fuel-basics https://pubmed.ncbi.nlm.nih.gov/15085273/ by Andreas Züttel https://www.sciencedaily.com/releases/2021/05/210506142118.htm https://seekingalpha.com/article/4392471-hydrogen-vs-natural-gas-for-electric-power-generation https://www.hydrogen.energy.gov/pdfs/hydrogen-program-plan-2020.pdf https://www.chemistryworld.com/features/hydrogen-storage-gets-real/3010794.article https://www.energy.gov/eere/fuelcells/hydrogen-storage-challenges https://www.twi-global.com/technical-knowledge/faqs/what-is-hydrogen-embrittlement
https://didionvessel.com/hydrogen-storage-tanks/
https://en.wikipedia.org/wiki/Electrolysis_of_water
https://www.theguardian.com/environment/2021/jan/15/western-australia-lng-plant-faces-calls-to-shut-down-until-faulty-carbon-capture-system-is-fixed
https://australia.chevron.com/-/media/australia/publications/documents/gorgon-co2-injection-project.pdf
https://agupubs.onlinelibrary.wiley.com/doi/full/10.1002/grl.50658
https://www.forbes.com/sites/rrapier/2020/06/06/estimating-the-carbon-footprint-of-hydrogen-production/?sh=5d6a103c24bd by Robert Rapier https://www.sciencedirect.com/science/article/abs/pii/S0013468613021622
https://www.thoughtco.com/metal-hydrides-2340044
https://www.quora.com/What-type-of-reaction-takes-place-in-the-Haber-process-exothermic-or-endothermic
https://research.csiro.au/hyresource/daintree-microgrid-project/
https://www.arena.gov.au/assets/2018/08/powering-daintree-sunverge.pdf
https://energycouncil.com/articles/hydrogen-hopefuls-five-groundbreaking-h2-projects/

