Fuelcells
Fuelcells
sources: www.nedstack.com/technology/fuel-cell-typeshttps://energy.gov/eere/fuelcells/types-fuel-cells
What is a Fuelcell?
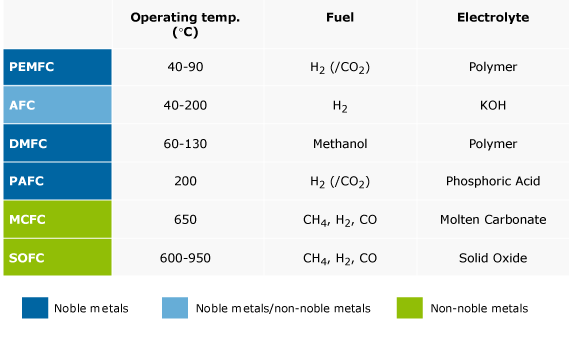
PEMFC type
AFC type
DMFC type
PAFC type
MCFC type
SOFC type
How does a fuelcell work?
What Is A Fuel Cell?
In principle, a fuel cell operates like a battery. Unlike a battery, a fuel cell does not run down or require recharging. It will produce energy in the form of electricity and heat as long as fuel is supplied.A fuel cell consists of two electrodes sandwiched around an electrolyte. Oxygen passes over one electrode and hydrogen over the other, generating electricity, water and heat.
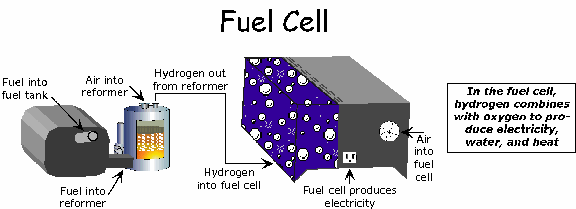
Hydrogen fuel is fed into the "anode" of the fuel cell. Oxygen (or air) enters the fuel cell through the cathode. Encouraged by a catalyst, the hydrogen atom splits into a proton and an electron, which take different paths to the cathode. The proton passes through the electrolyte. The electrons create a separate current that can be utilized before they return to the cathode, to be reunited with the hydrogen and oxygen in a molecule of water.
A fuel cell system which includes a "fuel reformer" can utilize the hydrogen from any hydrocarbon fuel - from natural gas to methanol, and even gasoline. Since the fuel cell relies on chemistry and not combustion, emissions from this type of a system would still be much smaller than emissions from the cleanest fuel combustion processes.
Characteristics of Fuel Cells
PEMFC
The electrolyte of the PEMFC consists of a proton-exchange membrane. The operating temperature is around 80°C. Cold start, below 0°C, is proven. For transport applications, the PEMFC is the fuel cell of choice. Although for stationary applications many alternatives exist, PEM fuel cells are being applied more and more, taking advantage of the impressive cost reductions in the last five years. Especially when fast start-up and load following dynamics are important and the supply of hydrogen is not an issue, PEM fuel cells offer a clear advantage over high temperature fuel cells. For those applications where reformed fuels are preferred, so-called high temperature PEMFC’s are being developed and applied. The rated power density of the PEMFC is nowadays 0.7 W.cm-2 and higher, depending on operating conditions
AFC
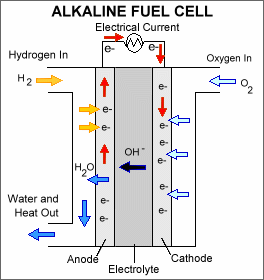
The electrolyte of the AFC consists of liquid Potassium Hydroxide. The operating temperature is around 80°C, but can be as high as 200°C. The AFC is currently being used for power generation on space crafts. The use of AFCs is limited because practically only pure hydrogen can be used as fuel. Air needs to be cleaned from CO2, which limits the application for terrestrial applications considerably. The power density of the AFC is in the range of 0.1 – 0.3 W.cm-2. A big potential advantage of alkaline fuel cells is that non-platinum containing electrodes can be used for both anode and cathode. In practice however, platinum is often used to enable higher power densities.
Recent developments are towards applying anion-exchange membranes, to get rid of the disadvantage of using a liquid electrolyte.
DMFC
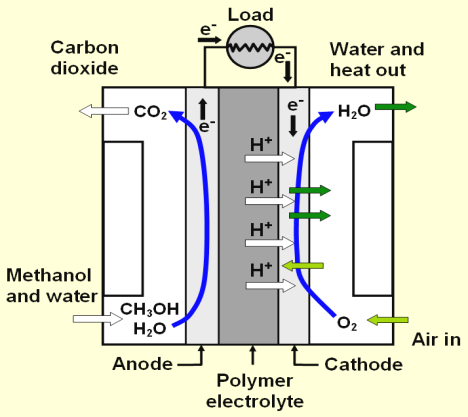
The direct methanol fuel cell is a variation of the PEMFC; it uses the same type of electrolyte. Instead of using hydrogen as fuel, methanol as solution in water is directly oxidized to CO2.The power density of the DMFC is considerably lower than that of the PEMFC. Maximum power densities, 0.25 W.cm-2 are obtained at a cell voltage as low as 0.4V. Compared to the PEMFC, high noble metal loadings are used, 1.2 mg.cm-2 or higher. The direct methanol fuel cell is mostly applied for low power applications, such as portable electronics, where they replace batteries, and as battery charger in the kW range for e.g. leisure and military applications.
PAFC
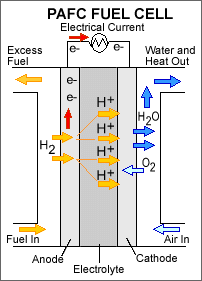
Liquid Phosphoric Acid is the electrolyte of the PAFC. The operating temperature is around 200°C. The PAFC can use reformate with CO concentrations up to 1-2%. The power density of the PAFC is in the range of 0.14 W.cm-2. Although the PAFC used to dominate the demonstration market in the 100 – 200 kW range, it seems to be overtaken by both PEMFC and MCFC systems in this segment. Typical applications are in the industrial and commercial combined heat and power.
MCFC
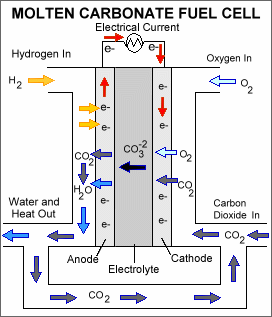
A molten mixture of lithium, sodium and potassium carbonate is used as the electrolyte in the MCFC. They also require carbon dioxide to be delivered to the cathode. As a consequence, contrary to other fuel cells, they require CO2 emission control. The operating temperature is between 600 and 700°C. Due to the high operating temperature, internal reforming of hydrocarbon fuels is possible. The power density of the MCFC is in the range of 0.1 – 0.12 W.cm-2. The power of MCFC systems is in the 50 kWe - 5 MWe range. Typical applications are in the industrial and commercial combined heat and power.
SOFC
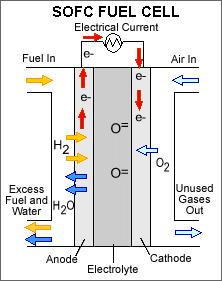
Yttrium stabilized Zirconia is generally used as the solid electrolyte in the SOFC. Depending on the electrolyte and the material composition of the electrodes, the SOFC can be operated between 600°C and 1000°C. Fuels ranging from hydrogen to natural gas and higher hydrocarbons can be used. The SOFC is mainly in development for stationary power generation for systems in the 1 kWe - 5 MWe range, although this range appears to be narrowed to systems of below 100 kW. While it used to be considered an important option for auxiliary power units on board of vehicles, this has not received much attention the last years. The power density of the SOFC is in the range of 0.15 – 0.7 W.cm-2.
How does a fuelcell work?
Fuel Cell Mode
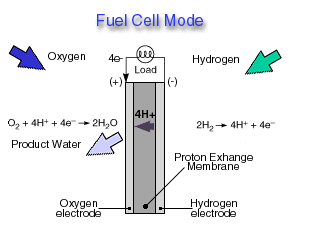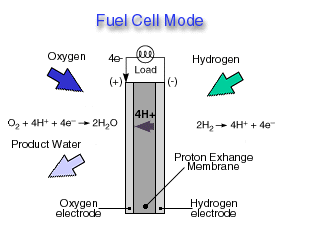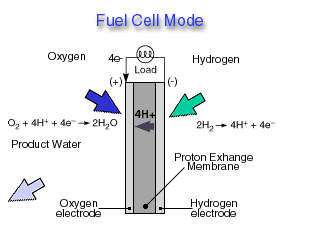
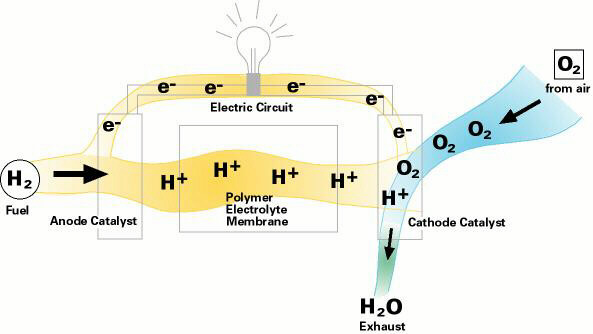
In a fuel cell, hydrogen gas from the fuel reacts electrochemically at one electrode and converts into protons and electrons. The protons move through the electrolyte to the other electrode, where they combine with oxygen from the air and with the electrons to form water, which is expelled from the cell as vapor. The involvement of hydrogen and oxygen in the two reactions - one releasing electrons and the other consuming them - yields electrical energy that is tapped across the electrodes for power, for example, to drive a motor.
Highly efficient fuel cells based on polymer electrolyte catalysts, known as proton-exchange membrane fuel cells, were developed by General Electric for the Gemini space program, but required large amounts of a costly platinum catalyst. The heart of the PEM fuel cell is a polymer membrane that has thin films of catalyst bonded on both its major surfaces, providing effective catalytic sites for the electrode processes.
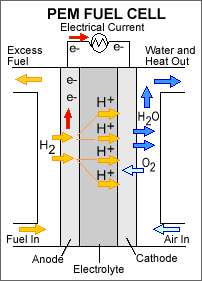
Highly efficient fuel cells based on polymer electrolyte catalysts, known as proton-exchange membrane fuel cells, were developed by General Electric for the Gemini space program, but required large amounts of a costly platinum catalyst. The heart of the PEM fuel cell is a polymer membrane that has thin films of catalyst bonded on both its major surfaces, providing effective catalytic sites for the electrode processes.Through a single electrochemical process, a fuel cell produces electricity, water, and heat using fuel and oxygen in the air . Water is the only emission when hydrogen is the fuel. As hydrogen flows into the fuel cell on the anode side (see Fuel Cell Mode figure), a platinum catalyst facilitates the separation of the hydrogen gas into electrons and protons (hydrogen ions) in a proton exchange membrane or PEM fuel cell.
The hydrogen ions pass through the membrane (the center part of a PEM fuel cell) and, again with the help of a platinum catalyst, combine with oxygen and electrons on the cathode side producing water. The electrons, which cannot pass through the membrane, flow from the anode to the cathode through an external circuit containing an electric load which consumes the power generated by the cell. The overall electrochemical process of a fuel cell is called "reverse hydrolysis," or the opposite of hydrolyzing water to form hydrogen and oxygen
.Electrolyzer Cell Mode
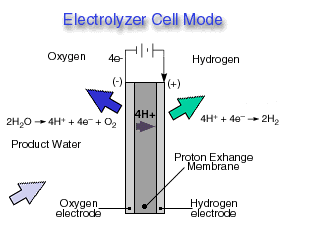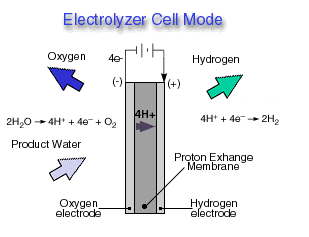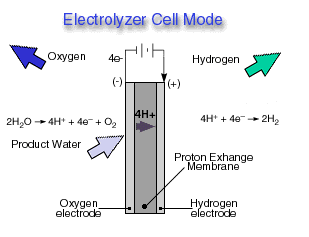
A reversible fuel cell can accomplish "hydrolysis" through the supply of electricity to the cell and a supply of water to the cathode. Only certain fuel cell types are reversible, that is, can also accomplish the electrochemistry associated with both the production of electricity from fuel and oxidant and the production of fuel and oxidant from water when supplied with electricity. The Reversible fuel cell concept is one that incorporates a reversible fuel cell that can accomplish both hydrolysis and reverse hydrolysis in the same cell. This allows one to consider the completely renewable production of electricity by using a renewable energy supply (e.g., solar, wind) to produce hydrogen and oxygen from water which can subsequently be used to produce electricity through the same fuel cell from the fuel and oxidant produced previously.

