Our Hydrogen Future
Our Hydrogen Future
Our hydrogen future: a very brief overview
also available in ReNew Magazine Issue 161
Hydrogen
from the electrolysis of water
Hydrogen
production from natural gas
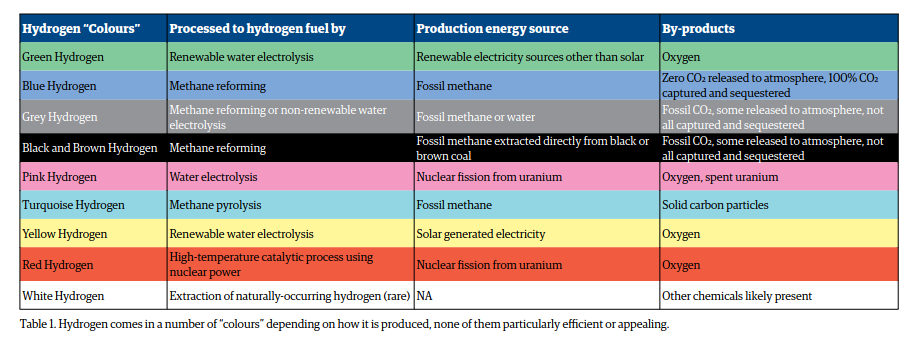
Types of Hydrogen:
Typical Waste
Sequestration Site
A
primer on carbon capture and sequestration (CCS)
So where do we stand?
see also Australia as a Major
Exporter of H2 Hydrogen - questions that need to be addressed
and Hydrogen Energy in Australia –
Green or Fossil Fuel ?Australia is being promoted on several levels as a future major exporter of hydrogen fuel. Not all technologies are equal and CCS (carbon capture and sequestration) is relied upon to contain some greenhouse gas emissions from hydrogen production.
The selling point of hydrogen fuel isthat when it is combusted, water is the only waste product. Hydrogen (H) is also the smallest atom and the simplest element. Containment during storage and transport is an issue.
Hydrogen by weight contains three times the energy of petrol, but because hydrogen is so much lighter (less dense), you need four times the volume of hydrogen to get the same energy as petrol. However, hydrogen has some advantages over fossil fuels:
- A fuel-cell car running on hydrogen is 60% to 80% efficient and achieves between five and 11 times the range (per kilogram of fuel) than a similar-size petrol-powered car (which is 18-30% efficient).
- Pipeline transport of hydrogen uses three times more volume (and therefore requires a 20% larger pipe diameter) to supply the same amount of energy as natural gas. This simple fuel must be extracted from compounds through processing.
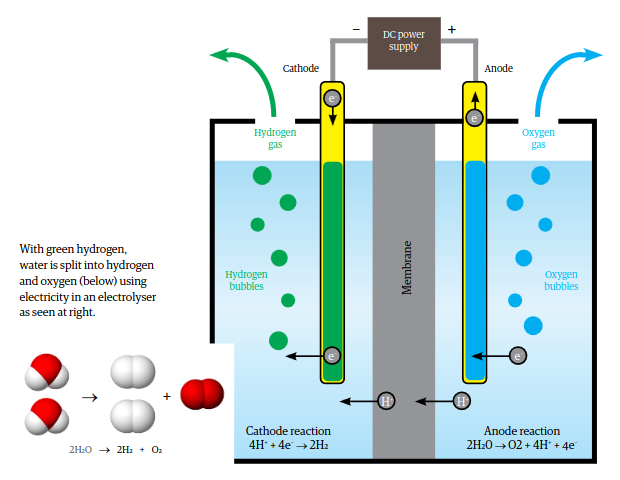
Hydrogen from the electrolysis of water
There are a number of different types of electrolysis devices on the market, with varying capacities and efficiencies.
To produce 1 kilogram of hydrogen at 100% efficiency:
- add 9 litres of fresh water to a container
- heat to 50 °C to 80 °C.
- insert two electrodes
- place a permeable membrane between the electrodes
- Connect DC electricity from renewable sources
- Oxygen (O2) bubbles off the positive electrode and
- Hydrogen (H2) bubbles off the negative electrode.
By comparison, an average Australian house consumes electricity amounting to 18 kWh per day.
Hydrogen production from natural gas
Put simply, the fossil fuel methane (natural gas) is extracted from underground.
At high temperature, using pure water, it is converted to hydrogen fuel and carbon dioxide waste.
The carbon dioxide waste is compressed to a liquid/gas state and injected into geological strata deep in the earth.
The hydrogen fuel is converted to liquid ammonia for export then converted back to hydrogen fuel or used to make fertiliser at its overseas destination.
About 7% to 18% of the energy contained in the H2 is lost in re-conversion.
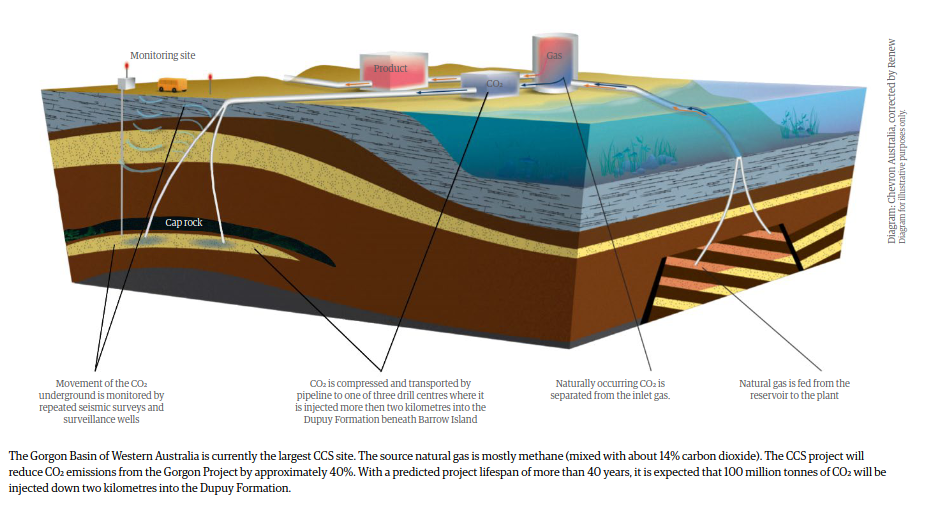
In the Gorgon Basin of Western Australia, the source natural gas is mostly methane mixed with about 14% carbon dioxide
--> Using the steam methane
reforming (SMR) process
A first step reacts steam with methane to produce hydrogen and carbon monoxide and then a second step reacts steam with carbon monoxide to produce hydrogen and carbon dioxide waste (called the watershift conversion). Greenhouse emissions come from both input power and the reaction.
--> Using Pyrolysis of (CH4) Methane
Pyrolysis is the thermal decomposition of materials at elevated temperatures in an inert atmosphere. Methane pyrolysis decomposition produces hydrogen with solid elemental carbon particles as a by-product and no direct carbon emissions.
Under development, it requires high temperatures to break the bonds of methane to produce hydrogen gas and solid carbon:
CH4 (gas) → C (solid) + 2H2 (gas)
The elemental carbon particles may be used in tyres, added to concrete, added to agricultural soil or buried in landfill. With further energy and processing it could possibly be converted to more valuable graphene.
Graphene is a material composed of pure carbon, similar to graphite but with characteristics that make it extraordinarily light and strong. A sheet of one square metre of graphene weighs 0.77 milligrams. Its strength is 200 times greater than that of steel and its density is similar to that of carbon fibre.
A first step reacts steam with methane to produce hydrogen and carbon monoxide and then a second step reacts steam with carbon monoxide to produce hydrogen and carbon dioxide waste (called the watershift conversion). Greenhouse emissions come from both input power and the reaction.
- Steam reformation: CH4 + H2O (+ heat) → CO + 3H2
- Water shift conversion: CO + H2O → CO2 + H2
- So, in total, we have: CH4 + 2H2O → CO2 + 4H2
- Assuming that there are only these reactions and that they are complete:
- Four molecules of H2 are created at the same time as one molecule of CO2
- A mol of H2 weighs 2 g and a mol of CO2 weighs 44 g, so...
- Producing 8 g of hydrogen fuel therefore automatically releases 44 g of waste CO2
- 1 kg of H2 therefore releases 5.5 kg of waste CO2 (at 100% efficiency)
- In practice, in a large hydrogen plant, due to heat losses and inefficiencies, 1 kg of H2 produces about 9.3 kg of waste CO2 and quantities of waste CH4.
--> Using Pyrolysis of (CH4) Methane
Pyrolysis is the thermal decomposition of materials at elevated temperatures in an inert atmosphere. Methane pyrolysis decomposition produces hydrogen with solid elemental carbon particles as a by-product and no direct carbon emissions.
Under development, it requires high temperatures to break the bonds of methane to produce hydrogen gas and solid carbon:
CH4 (gas) → C (solid) + 2H2 (gas)
The elemental carbon particles may be used in tyres, added to concrete, added to agricultural soil or buried in landfill. With further energy and processing it could possibly be converted to more valuable graphene.
Graphene is a material composed of pure carbon, similar to graphite but with characteristics that make it extraordinarily light and strong. A sheet of one square metre of graphene weighs 0.77 milligrams. Its strength is 200 times greater than that of steel and its density is similar to that of carbon fibre.
Types of Hydrogen:
Typical Waste Sequestration Site
A primer on carbon capture and sequestration (CCS)
The idea behind CCS is to capture the waste CO2 and inject it deep into the earth so it can’t pollute the atmosphere or acidify the oceans.--> Where to store the CO2
A good geologic storage formation for CCS must meet four main criteria.
- First, for the host formation to receive the CO2, it must be porous (have spaces) with good permeability (the spaces are connected)
- Second, the host formation should be at a depth of about two to three kilometres, with pressures of 200 to 300 bar and temperatures of 60 °C to 100 °C. This keeps the CO2 in a more compact liquid/gas state
- Third, it is essential that above the host formation is a continuous layer of impermeable caprock such as shale. The liquid/gas CO2 is less dense than the ground water and will continually try to migrate upwards if it is not blocked
- Finally, the formation must be able to store large volumes of CO2.
Four main mechanisms work together to trap the CO2 in the formation:
- structural trapping,
- capillary trapping,
- solubility trapping, and
- mineral trapping
Structural trapping keeps CO2
beneath the formation’s impermeable caprock. Rising CO2
encounters the caprock, stopping vertical migration. Lateral migration
is contained if the caprock is shaped like an inverted bowl or hat
Capillary trapping is a function of water and CO2 competing to move though the small pores between sand grains. The more dense water traps the less dense CO2 in the little tunnels.
Solubility trapping: Some CO2 dissolves into the ground water. Since CO2 saturated water is denser than unsaturated water, it will sink, bringing more unsaturated water in contact with the CO2
Mineral trapping is a very slow process where CO2 combines with other rock elements to form solid insoluble minerals.
Capillary trapping is a function of water and CO2 competing to move though the small pores between sand grains. The more dense water traps the less dense CO2 in the little tunnels.
Solubility trapping: Some CO2 dissolves into the ground water. Since CO2 saturated water is denser than unsaturated water, it will sink, bringing more unsaturated water in contact with the CO2
Mineral trapping is a very slow process where CO2 combines with other rock elements to form solid insoluble minerals.
--> How long will the CO2 stay underground?
The crust of the earth is dynamic. It erupts, quakes, moves, bends, breaks, fractures, faults and erodes over time. Eventually over time the sequestered CO2 will escape. The IPCC (Intergovernmental Panel on Climate Change) estimates:
- after 100 years, 90% to 99% of sequestered CO2 remains in place, and
- after 1000 years, sequestered CO2 exceeds 66% to 90%
- the IPCC will not and cannot guarantee that a fluid injected in the ground will never come out.
So where do we stand?
Hydrogen fuel production from the electrolysis of water is a battery technology which uses lots of fresh water and lots of electricity. It is useful as a fuel “beyond the grid” for bulk transport like ships, trains, trucks and aircraft where bulky fuel tanks are not a design issue. It may have a place in adding power to the grid in times of peak demand, but this begs the question, in a country where fresh water is scarce, why not use better battery technologies? Hydrogen from natural gas is a processed fossil fuel. Extraction, conversion and injection and sequestration processes do not capture all greenhouse gases. How much CO2 and CH4 escapes is currently obscured— it would appear at least 15% and more realistically 60% is lost before sequestration. After sequestration, a portion of that which is captured and sequestered will eventually come back through fugitive emissions.
Our hydrogen future?
It’s a gamble whether this kicking the can down the road buys humankind 100 or 10,000 years
Main Sources
Jin Zhong Zhang, Jinghong Li, Yat Li, Yiping Zhao, 2014, Hydrogen Generation, Storage, and Utilization, co-published by John Wiley & Sons, Inc., Hoboken, New Jersey; and ScienceWise Publishing ISBN 978-1-118-14063-5
Garcia L. 2015, Hydrogen production by steam reforming of natural gas and other nonrenewable feedstocks, in Compendium of hydrogen energy, Vol. 1 Hydrogen production and purification, éd. Woodhead Publishing, Kidlington
Herzog, Howard J. 2018, Carbon Capture, The MIT Press Essential nowledge Series, The MIT Press, Cambridge, MA ISBN 9780262535755
Nuttall William J. , Adetokunboh T. Bakenne, 2020, Fossil Fuel Hydrogen Technical, Economic and Environmental Potential, Springer Nature Switzerland AG, ISBN 978-3-030-30907-7 ISBN 978-3-030-30908-4 (eBook)
Havercroft Ian, Macrory Richard & Stewart Richard, 2018, Carbon capture and storage : emerging legal and regulatory issues, Second edition, Hart Publishing, Portland, Oregon, ISBN 9781509909605
www.industry.gov.au/sites/default/files/October%202021/ document/australias-long-term-emissions-reduction-plan.pdf Australian CCS sites map pg 54
climatechangeconnection.org/emissions/co2-equivalents
energy.gov/eere/fuelcells/hydrogen-production-natural-gas- reforming
en.wikipedia.org/wiki/Hydrogen_production
forbes.com/sites/rrapier/2020/06/06/estimating-the-carbon- footprint-of-hydrogen-production
whatispiping.com/colors-of-hydrogen
large.stanford.edu/courses/2010/ph240/chen1
hydrogene.discoverthegreentech.com/en/production/steam- methane-reforming
aph.gov.au/About_Parliament/Parliamentary_Departments/ Parliamentary_Library/pubs/rp/rp9899/99rp05#COMP anz.com/content/dam/anzcom/pdf/institutional/reports/ hydrogen-handbook.pdf
d3n8a8pro7vhmx.cloudfront.net/lockthegate/pages/6525/ attachments/original/1581635517/Fact_sheet_A_tale_of_two_ fuels_-_vs1._pdf.pdf

