Minerals
Minerals
source http://ga.gov.auhttp://www.galleries.com/Silicates
http://academic.brooklyn.cuny.edu/geology/grocha/mineral/cleavage.html
http://butane.chem.uiuc.edu/pshapley/Environmental/L26/2.html
http://www.tulane.edu/~sanelson/eens211/silicate_structures08.htm

Mineral Identification Basics
- Colour
- Streak
- Lustre
- Transparency
- Form
- Habit
- Crystal Systems
- Cleavage
- Fracture
- Hardness
- Density
- Magnetism
- Effervescence
- Birefringence
- Fluorescence
- Taste
- Odour
- Sparkle
- Feel
- Conductivity
- Acid Reaction
- Tenacity
Mineral Groups
Silicates
- Nesosilicates (single tetrahedrons)
- Sorosilicates (double tetrahedrons)
- Inosilicates (single and double chains)
- Cyclosilicates (rings)
- Phyllosilicates (sheets)
- Tectosilicates (frameworks)
Sulphates
Sulphides
Table of common non-metallic minerals and their
properties
Table of common metallic minerals and their
properties
click
here to open a large mineral identification flow chart in a new
window...
List of
common minerals and their images...
see
also E-Learning - Minerals...
see also
Petrology - Mineral Optics...

Mineral Identification Basics
Colour
The colour of a mineral is a result of the mineral's light absorbing and light reflecting properties. These may vary greatly in vitreous minerals with the presence of traces of impurities. Colour is therefore not always an indication of identity in a vitreous specimen, although it is a more reliable indicator with opaque minerals.
An excellent example of the above is quartz. Six different varieties of quartz are each a different characteristic colour despite having identical chemical compositions (SiO2):
Rock Crystal - colourless
Amethyst - purple
Citrine - yellow, to orange-brown
Smokey Quartz - brown or grey
Rose Quartz - pink
Milky Quartz - white
It is also worth remembering that completely different minerals may be the same colour.
Streak
The streak of a mineral is the colour of it's powder when rubbed along an
unglazed porcelain plate (streak-plate) and may be different from the
colour of the mineral itself. 
Powder may also be produced by scratching the mineral with a knife. The streak of any given mineral is consistent for that mineral despite any differences in colour. The six different varieties of quartz above all have the same white streak.
Lustre
The mineral's appearance due to the amount and quality of light reflected
from it's surfaces. Depending on the quality of light a mineral
reflects it may appear: Adamantine - the lustre of diamond
Vitreous - the lustre of broken glass, e.g.. quartz
Subvitreous - as vitreous, less well developed
Resinous - the lustre of resin, e.g.. amber and opal
Pearly - the lustre of pearl
Silky - the lustre of silk in fibrous minerals such as satin spar gypsum
Metallic - the lustre of metal
Submetalic - as metallic, poorly displayed

Depending on the quantity or intensity of light a mineral reflects it may appear:
Splendent
Shining
Dull
Transparency
- If an object can be seen with a clear outline through a mineral then
that mineral is transparent.
- If an object viewed through a mineral can be seen with a indistinct
outline then the mineral is said to be subtransparent.
- If a mineral cannot be seen through, but is transmitting light then
that mineral is said to be translucent.
- A mineral that does not transmit light is termed opaque.
Form
The form of a crystal is dependant upon the conditions under which it
grew. For example growth may have occurred outwards into a melt
unhindered or it may have been restricted by the presence of other solid
matter. The following terms are used to describe form:
Crystallized - the mineral occurs as well developed crystals
Crystalline - the mineral occurs as an aggregate of confused, imperfect crystals which hindered each other's formation during growth. These minerals often have a granular, sparkling appearance due to light reflected from the small crystal faces.
Cryptocrystalline or Microcrystalline - crystals are very small and are hidden from the naked eye, but show under a microscope.
Glass - random arrangement of atoms; no crystal structure. A substance which cooled so rapidly that crystals did not have time to form. The result may be thought of as a stiff, brittle supercooled liquid.
Habit
The habit of a specimen (the shape of it's crystals) is greatly affected
by the conditions under which the crystals grew. It is quite common
for a mineral to have many different habits. The terms used to describe a specimen's habit are split into two groups;
(1) the habit of crystals,
(2) the habit of crystal aggregates.

1. Crystal Habits
Prismatic - crystal is elongated along one axis
Tabular - Broad, flat crystals
Acicular - needle-like crystals
Bladed - the shape of a knife blade
Fibrous - fine thread fibres as in asbestos and satin spar gypsum
Foliaceous - composed of thin separate leaves (lamellae) as in mica.
Lamellar - separable into individual plates or lamellae
Reticulate - cross-mesh pattern
Scaly - small plates
Individual crystals may be described by their shape i.e.. cubic, hexagonal elongated (prismatic),
lozenge, rhombohedral, octohedral, etc.
2. Crystal Aggregate Habits
Amygdales - are spherical aggregates infilling vesicles in 'amygdaloidal' rocks.
Massive columnar - such as in stalactites and stalagmites aggregates.
Nodular - such as flint nodules in chalk
Granular or Saccharoidal - grains, may range from coarse to fine. Saccharoidal means 'sugar-like'.
Mammilated - similar to reniform (below), but more spherical outer surfaces. e.g.. malachite.
Reniform - 'kidney-shaped' rounded outer surface. e.g. haematite / hematite
A mineral with no crystal or aggregate shape is a glass.
Crystal Systems
- many minerals form crystals as they cool. There are six systems of crystals based on their geometry.For each system listed below there are some examples of minerals belonging to that system.
"click on any link to see an image of the mineral"

Triclinic Minerals include: albite, amblygonite, anapaite, andesine, anorthite, anothoclase, astrophyllite, axinite, babingtonite, baumhauerite, bustamite, bytownite, ceruleite, chabazite-(Ca), chalcanthite, chalcosiderite, chloritoid (triclinic), collinsite, copiapite, cornubite, cuprosklodowskite, cylindrite, diadochite, emmonsite, fairfieldite, ferroaxinite, fiedlerite, fluckite, frankeite, gageite-1tc, gordonite, halloysite, hydrodresserite, inesite, jamesite, kermesite, kyanite, labradorite, lengenbachite, ludlockite, manganaxinite, manganbabingtonite, metakottingite, microcline, miserite, montebrasite, nambulite, nealite, oligoclase, paradamite, paravauxite, pectolite, picropharmacolite, planerite, pyrophyllite, pyroxmangite, rhodonite, sapphirine, serandite, serendibite, serpentine, strunzite, symplesite, tarbuttite, tinzenite, turquoise, ulexite, ussingite, vauxite, weloganite, wollastonite, amesite, donnayite-(Y), epistilbite, epistolite, hilgardite, kaolinite, leucophanite, magnesio-axinite, murmanite, nacaphite, nefedovite, tundrite and tyretskite.
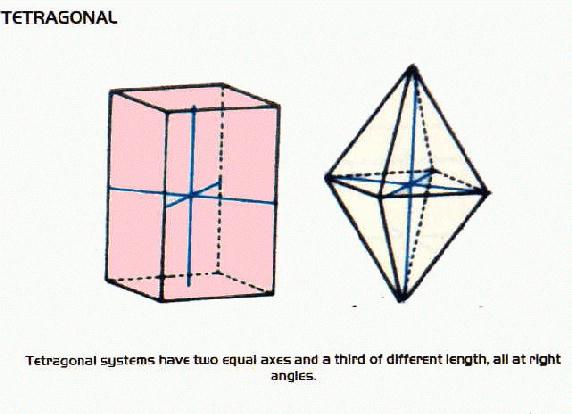
Tetragonal Minerals include: apophyllite, autunite, meta-autunite, torbernite, meta-torbernite, xenotime, carletonite, plattnerite, zircon, hausmannite, pyrolusite, thorite, anatase, vesuvianite, rutile and cassiterite
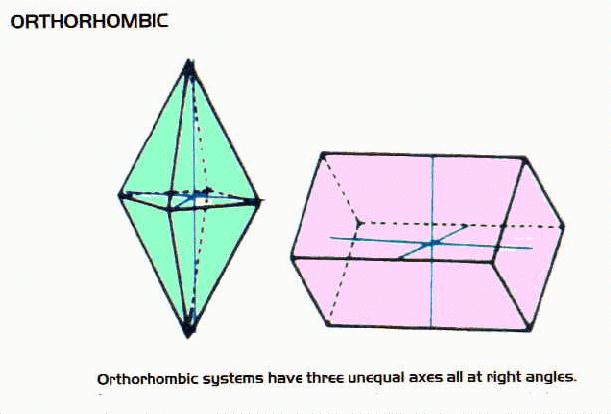
Orthorhombic Minerals include: barite group minerals as well as sulfur, staurolite, olivine, andalusite, members of the aragonite group minerals, marcasite, topaz, brookite, enstatite, anthrophyllite, sillimanite, zoisite, adamite, danburite, cordierite, wavellite
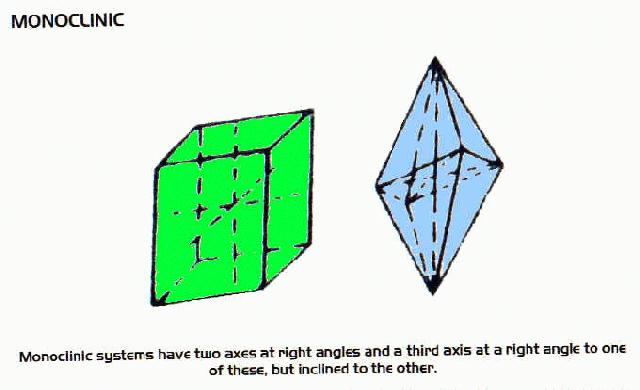
Monoclinic Minerals include: acanthite, actinolite, aegirine, allanite, annabergite, arfvedsonite, arsenopyrite, augite, aurichalcite, azurite, bannisterite, beryllonite, biotite, borax, boulangerite, brazilianite, brochantite, butlerite, calaverite, carnotite, catapleiite, caledonite, celsian, chalcocite, charoite, chondrodite, chrysotile serpentine, clinochlore, clinoclase, clinoptilolite, colemanite, cookeite, cornwallite, creedite, crocoite, cryolite, cryptomelane, cummingtonite, datolite, diopside, dufrenite, edenite, epidote, erythrite, esperite, euclase, fluorrichterite, gadolinite, gaylussite, gibbsite, glauberite, glauconite, graftonite, gypsum, harmotome, hedenbergite, hessite, heulandite, hodgkinsonite, hornblende, howlite, hubnerite, hydroboracite, hydromagnesite, hydrozincite, illite, jadeite, jamesonite, jordanite, kernite, kidwellite, kieserite, kinoite, kottingite, kovdorskite, ktenasite, lamprophyllite, lanarkite, langbanite, laumontite, lazulite, leadhillite, legrandite, leucosphenite, linarite, liroconite, ludlamite, malachite, manganite, melanterite, monazite, montmorillonite, muscovite, olivenite, orpiment, orthoclase, palygorskite, papagoite, pargasite, phillipsite, phlogopite, polybasite, polylithionite, pseudomalachite, psilomelane, pyrrhotite, realgar, richterite, riebeckite, romanechite, rosasite, roselite, sanidine, sauconite, semseyite, sklodowskite, sphene, spodumene, staurolite, stilbite, stringhamite, sussexite, sylvanite, synchysite, tainiolite, talc, tenorite, thomsenolite, titanite, tremolite, trona, vermiculite, veszelyite, vivianite, volborthite, whewellite, whiteite, wohlerite, wolframite, xonotlite, zinnwaldite, zippeite and zirconolite-2M
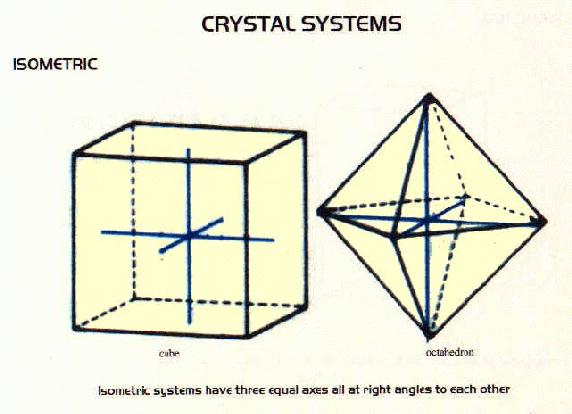
Isometric Minerals include: fluorite, galena, diamond, copper, iron, lead, platinum, silver, gold, halite, bromargyrite, chlorargyrite, moschellandsbergite, murdochite, osbornite, periclase, pollucite, villiaumite, pyrochlore, thorianite, the garnet group, uraninite, most members of the spinel group, pentlandite, sylvite, analcime
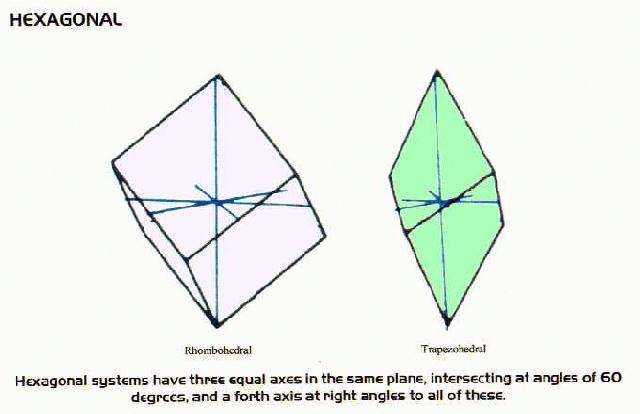
Hexagonal Minerals include: calcite group as well as corundum, hematite, bismuth, antimony, sturmanite, brucite, arsenic, soda niter, chabazite, millerite, quartz, tellurium berlinite, cinnabar, tourmaline group, pyrargyrite, jarosite, natrojarosite, alunite, proustite,dolomite group, ankerite, ilmenite, dioptase, willemite, phenakite,
Amorphous Minerals
-show no crystal structureAmorphous Minerals include: amber, limonite, obsidian, opal, petroleum, shungite, tektite
"click on any link to see an image of the mineral"
Cleavage
Cleavage is the tendency of a mineral to split in certain preferred
directions when struck. These directions are parallel to sheets of
atoms in the mineral's atomic lattice. 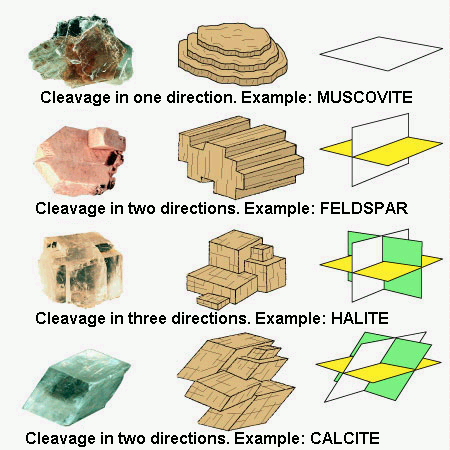
- Cleavage is described in terms of: the ease of cleavage
- the number and orientations of cleavage planes. For example: Gypsum has 'easy' cleavage in one direction
Fluorite has a cubic habit, but it has four cleavage directions which cut across it's corners to leave an octahedral core. Therefore fluorite has octahedral cleavage.
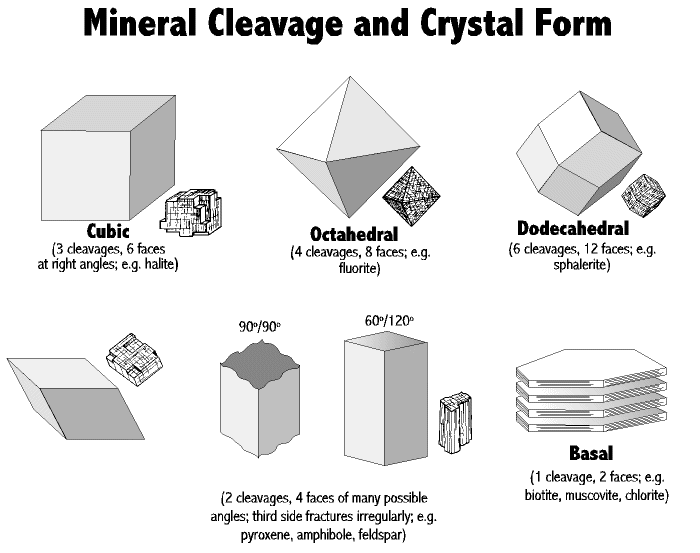
Fracture
The fracture of a mineral is how it breaks other than along cleavage
planes. The fracture may be described as: Conchoidal - a
'shell-like', convex or concave fracture displaying curved fracture or
undulation rings concentric to the point of impact and lines or fractures
radial from the point of impact, as in quartz,
flint and obsidian. 
Even - a flat fracture, as in chert
Uneven - a rough fracture surface. This is the most common type of fracture.
Hackly - jagged sharp ridges, such as in native copper.
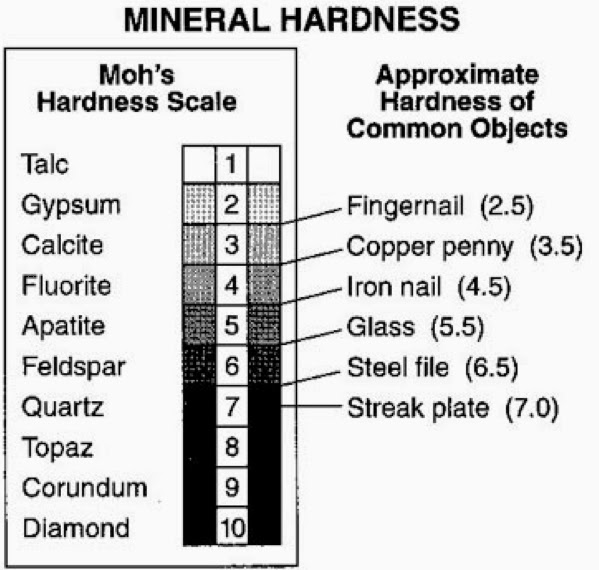
Hardness
The hardness of a mineral is measured on Moh's scale. The
scale lists hardness values from 1 to 10. The numbers may be treated
as relative values except for diamond; i.e. fluorite(4)
is twice the hardness of gypsum(2). Diamond(10)
is about ten times the hardness of corundum(9).
Each value has a corresponding mineral of thathardness. Therefore
the hardness of a mineral can be tested relative to the minerals on Moh's
scale by scratching them with those minerals and other household items of
known hardness. Moh's scale of hardness
Density
The relative density of a mineral is its mass divided by it's volume. The specific gravity of a mineral is it's mass divided by the mass of an equal volume of water. In the field it is adequate to simply 'heft' a specimen to determine whether it is of low, high or moderate weight compared to it's size.
Silicates and other non-metallic minerals are the least dense with SGs of 2.5 to 3.5
Metallic minerals are denser with SGs from 5 upwards (typically 5 to 8). Gold has an SG of 19 to 20.
Vitreous minerals are usually 'light' and metallic usually dense, but be aware that there are always exceptions to the rule.
Table Showing Specific Gravities of Minerals
| Low SG | Medium SG | High SG | Very High SG |
|---|---|---|---|
| (all vitreous) | (all metallic) | (metal ores) | |
| gypsum | muscovite | barite | cassiterite |
| halite (rock salt) | biotite | malachite | galena |
| graphite | calcite | sphalerite | |
| fluorite | chalcopyrite | ||
| hornblende | haematite/ hematite | ||
| augite | magnetite | ||
| orthoclase | pyrite | ||
| plagioclase | |||
| olivine | |||
| quartz |
Magnetism
Magnetite and pyrrhotite are magnetic and will be affected by a bar
magnet. Other iron minerals are magnetic to a lesser extent, but
cannot be tested by an ordinary magnet in the field. Large
iron-bearing masses may affect the orientation of compass needles. A
petrology lecturer described how he once stopped for lunch on a large
magnetite-bearing outcrop and then set off in completely the wrong
direction and wasted the rest of the day.Is this mineral magnetic (try
using a compass), or is it attracted by a magnet? This property is
characteristic of Magnetite. Effervescence
This one is most popular with the kiddies as well as the new geology student (welcome). When some minerals are exposed to acids, they begin to fizz. This is a great method you can use to identify the mineral calcite (see TASTE). You can also use this one to detect the presence of calcite in rocks.
Birefringence

This is also known as double refraction.
Birefringent minerals split the light into two different rays which gives the illusion of double vision in this Iceland Spar Calcite.
Fluoresence

Some minerals display what is called the phenomenon of photoluminescence. This basically means that they "glow" when exposed to UV light (black light). The above mineral (opal) is demonstrating fluoresence. Also, the mineral Fluorite is often strongly fluorescent. Do you see a connection? Fluorite --> Fluoresence
Taste
This will quickly identify the mineral halite (salt). If you are new to this process you must use this one with caution, as you never know what the unknown may be. Often, you may need to resort to this method (until you more fully understand other identifying traits) to differentiate halite from calcite. If you do taste the sample (especially in a class environment) you should realize that it has been handled by and probably tasted by hundreds of others.Odour
Pyrite, sphalerite and chalcopyrite give a sulphurous 'rotten egg' smellwhen struck or rubbed on a streak plate. haematite and limonite may give off
an 'earthy' smell (the smell of damp earth) when breathed upon.
Sparkle
Pyrite sparkes when struck with a geological
hammer. I have also experienced this effect with haematite.
Feel
Crystalline minerals will feel rough. Talc and serpentine often feelunctuous (greasy) or soapy. Graphite and satin spar gypsum may
feel smooth, unctous or soapy. Graphite is a good conductor of
heat and will therefor feel cold.
Conductivity
Graphite is also a good conductor of electricity (it is used a brushes onelectric motors), but this property would not be tested in the field.Graphite is a good example
Acid Reaction
Carbonate minerals react with dilute hydrochloric acid: Calcite effervesces strongly in dil. HClMalachite also reacts strongly
Dolomite reacts weakly in warm dil. HCl or if scratched
to produce a little powder prior to applying the acid
Siderite reacts weakly
Tenacity
Tenacity describes how the mineral behaves when subjected to deformation: Brittle - The minerals breaks or crumbles easily, such as fluorite.- Ductile - the mineral can be drawn into thin wires.
- Elastic - the mineral can be deformed by force, but returns to it's
original shape when the deforming force is removed.
- Flexible - the mineral can be bent or deformed by force and remains deformed when the force is removed.
- Malleable - Can be flattened into sheets with hammering, such as
native gold, silver and copper.
Mineral Groups
Minerals are divided into the following groups:- Carbonates - carbonate minerals contain CO3 (carbon and oxygen) combined with other elements. Calcite is the most common carbonate mineral
- Halides - halides are mostly salts and include elements like chlorine and fluorine. Common halides are halite (common salt) and fluorite
- Native Elements - native elements are minerals that form as individual elements. Copper and gold are examples of these
- Oxides - oxides, as one can imagine, are made up of oxygen and one or more metal. Hematite is an oxide.
- Silicates - silicates make up over 90% of common rock-forming minerals and contain silicon and oxygen (the two most abundant elements in the earth's crust).
- Sulfates - sulfates contain SO4 (sulfur and oxygen) combined with other elements. Gypsum is a sulfate.
- Sulfides - sulfides contain sulfur and metal. Common sulfide minerals are galena and pyrite.
Carbonates
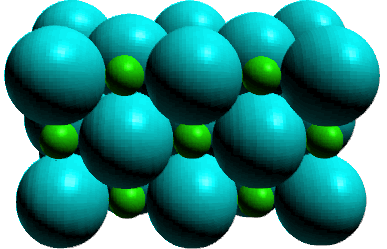
Carbonate Minerals are based on the salt calcium carbonate.
Calcium Carbonate ionic bonds between the calcium +2 cations and the carbonate -2 anions. There are strong, covalent bonds within the CO32- group.
The carbonate minerals have a structure that is similar to the cubic close packed structure found in halite (NaCl) where the Na cations are replaced by divalent cations (Ca, Mg, Fe, Mn, Sr, Ba, Pb, etc.) and the Cl anions are replaced by CO32- polyatomic trigonal planar ions. Think of the ions as being located on two face-centered cubic lattices that interpenetrate one another.
Minerals in the carbonate group differ do not have true cubic close packed symmetry because anions and the cations differ significantly in size. They are distorted from this type of crystal form.
Limestone, a sedimentary rock, becomes marble from the heat and pressure of metamorphic events. Calcite is even a major component in the igneous rock called carbonatite.
Dolomite, CaMg(CO3)2, is a common sedimentary rock-forming mineral that can be found in massive beds several hundred feet thick. They are found all over the world and are quite common in sedimentary rock sequences. All dolomite rock was initially deposited as calcite/aragonite rich limestone, but during a process call diagenesis the calcite and/or aragonite is altered to dolomite.
All carbonates have some water solubility and dissolve readily in acidic water. They dissolve in acidic water and can recrystallize from the water. Metal ions are frequently trapped in the lattice spaces during crystallization.
This leads to carbonates with a variety of colors and crystal forms.
Carbonic acid-rich water forms caves in limestone. When the water table is high, carbonic acid-rich water dissolves the limestone (calcite). Later when the water table drops, a void filled with air is formed. Smaller amounts of water rich in Ca2+ and HCO3- may continue to flow through the void. These waters decrease the CO2 partial pressure in the atmosphere of the cave and aqueous CO2 is released into the gas phase. This increases the pH and drives the precipitation of calcite and formation of stalagmites, stalactites and other cave features. Salts containing the carbonate anion decompose with loss of carbon dioxide. This is an endothermic reaction and produces metal oxide materials. The carbonates are more stable, in general, with larger cations.
Halides

Halides are formed by combining a metal with one of the five halogen elements, chlorine, bromine, fluorine, iodine, and astatine.
Many of these compounds will dissolve in water and are often associated with evaporating seas.
Halite (NaCl) or rock salt is the most common.
Other halides minerals include: Fluorite CaF2 or calcium fluoride and Sylvite KCl or potassium chloride
Native Elements

Minerals found naturally and composed of just one element - examples gold, silver, copper
Oxides
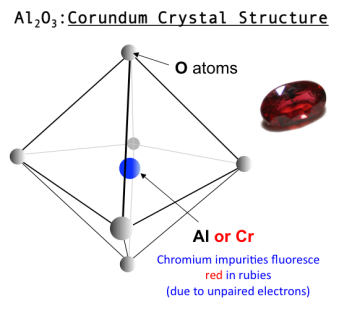
The oxide mineral class includes those minerals in which the oxide anion (O2−) is bonded to one or more metal ions.
It includes: gemstones such as Corundum (Aluminum Oxide), Chrysoberyl (Beryllium Aluminum Oxide), Spinel (Magnesium Aluminum Oxide)
metallic ores such as Hematite (Iron Oxide), Ilmenite (Iron Titanium Oxide), Magnetite (Iron Oxide), Chromite (Iron Chromium Oxide)
The hydroxide bearing minerals are typically included in the oxide class
Hydroxide minerals include: Limonite (Hydrated Iron Oxide Hydroxide), Manganite (Manganese Oxide Hydroxide), Gibbsite (Aluminum Hydroxide)
Silicates
Silicates are the largest, the most interesting, and the most complicated class of minerals by far. Approximately 30% of all minerals are silicates and some geologists estimate that 90% of the Earth's crust is made up of silicates. With oxygen and silicon the two most abundant elements in the earth's crust, the abundance of silicates is no real surprise. The basic chemical unit of silicates is the (SiO4) tetrahedron shaped anionic group with a negative four charge (-4). The central silicon ion has a charge of positive four while each oxygen has a charge of negative two (-2) and thus each silicon-oxygen bond is equal to one half (1/2) the total bond energy of oxygen. This condition leaves the oxygens with the option of bonding to another silicon ion and therefore linking one (SiO4) tetrahedron to another and another, etc..The complicated structures that these silicate tetrahedrons form is truly amazing. They can form as single units, double units, chains, sheets, rings and framework structures. The different ways that the silicate tetrahedrons combine is what makes the Silicate Class the largest, the most interesting and the most complicated class of minerals.
The Nesosilicate Subclass (single tetrahedrons) 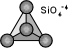
The simplest of all the silicate subclasses, this subclass includes all silicates where the (SiO) tetrahedrons are unbonded to other tetrahedrons. In this respect they are similar to other mineral classes such as the sulfates and phosphates. These other classes also have tetrahedral basic ionic units (PO4 & SO4) and thus there are several groups and minerals within them that are similar to the members of the nesosilicates. Nesosilicates, which are sometimes referred to as orthosilicates, have a structure that produces stronger bonds and a closer packing of ions and therefore a higher density, index of refraction and hardness than chemically similar silicates in other subclasses. Consequently, There are more gemstones in the nesosilicates than in any other silicate subclass. Below are the more common members of the nesosilicates. See the nesosilicates' page for a more complete list.
- Andalusite (Aluminum Silicate)
- Chloritoid (Iron Magnesium Manganese Aluminum Silicate Hydroxide)
- Datolite (Calcium Boro-Silicate Hydroxide)
- Euclase (Beryllium Aluminum Silicate Hydroxide)
- Fayalite (Iron Silicate)
- Forsterite (Magnesium Silicate)
- Gadolinite (Yttrium Iron Beryllium Silicate)
- The Garnet Group:
- Almandine (Iron Aluminum Silicate)
- Andradite (Calcium Iron Silicate)
- Grossular (Calcium Aluminum Silicate)
- Pyrope (Magnesium Aluminum Silicate)
- Spessartine (Manganese Aluminum Silicate)
- Uvarovite (Calcium Chromium Silicate)
- Howlite (Calcium Boro-Silicate Hydroxide)
- Humite (Magnesium Iron Silicate Fluoride Hydroxide)
- Kyanite (Aluminum Silicate)
- Olivine (Magnesium Iron Silicate )
- Phenakite (Berylium Silicate )
- Sillimanite (Aluminum Silicate)
- Sphene or Titanite (Calcium Titanium Silicate)
- Staurolite (Iron Magnesium Zinc Aluminum Silicate Hydroxide)
- Thorite (Thorium Uranium Silicate)
- Topaz (Aluminum Silicate Fluoride Hydroxide)
- Uranophane (Hydrated Calcium Uranyl Silicate)
- Willemite (Zinc Silicate)
- Zircon (Zirconium Silicate)
Sorosilicate Subclass (double tetrahedrons) 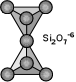
Sorosilicates have two silicate tetrahedrons that are linked by one oxygen
ion and thus the basic chemical unit is the anion group (Si2O7) with a
negative six charge (-6). This structure forms an unusual hourglass-like
shape and it may be due to this oddball structure that this subclass is
the smallest of the silicate subclasses. It includes minerals that may
also contain normal silicate tetrahedrons as well as the double
tetrahedrons. The more complex members of this group, such as Epidote,
contain chains of aluminum oxide tetrahedrons being held together by the
individual silicate tetrahedrons and double tetrahedrons. Most members of
this group are rare, but epidote is widespread in many metamorphic
environments. Below are the more common members of the sorosilicates. See
the sorosilicates' page for a more complete list. - Bertrandite (Beryllium Silicate Hydroxide)
- Danburite (Calcium Boro-Silicate)
- The Epidote group
- Allanite (Yttrium Cerium Calcium Aluminum Iron Silicate Hydroxide)
- Clinozoisite (Calcium Aluminum Silicate Hydroxide)
- Epidote (Calcium Iron Aluminum Silicate Hydroxide)
- Zoisite (Calcium Aluminum Silicate Hydroxide)
- Hemimorphite (Hydrated Zinc Silicate Hydroxide)
- Ilvaite (Calcium Iron Silicate Hydroxide)
- Idocrase or Vesuvianite (Calcium Magnesium Aluminum Silicate Hydroxide)
Inosilicate Subclass (single and double chains)
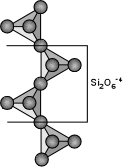
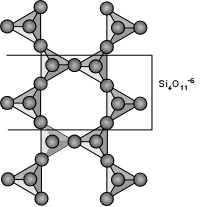
This subclass contains two distinct groups: the single chain
and double chain silicates. In the single chain group the tetrahedrons
share two oxygens with two other tetrahedrons and form a seemingly
endless chain. The ratio of silicon to oxygen is thus 1:3. The
tetrahedrons alternate to the left and then to the right along the line
formed by the linked oxygens although more complex chains seem to
spiral. In cross section the chain forms a trapezium and this shape
produces the angles between the crystal faces and cleavage directions.In the double chain group, two single chains lie side by side so that all the right sided tetrahedrons of the left chain are linked by an oxygen to the left sided tetrahedrons of the right chain. The extra shared oxygen for every four silicons reduces the ratio of silicons to oxygen to 4:11. The double chain looks like a chain of six sided rings that might remind someone of a child's clover chain. The cross section is similar in the double chains to that of the single chains except the trapezium is longer in the double chains. This difference produces a difference in angles. The cleavage of the two groups results between chains and does not break the chains thus producing prismatic cleavage. In the single chained silicates the two directions of cleavage are at nearly right angles (close to 90 degrees) forming nearly square cross sections. In the double chain silicates the cleavage angle is close to 120 and 60 degrees forming rhombic cross sections making a convenient way to distinguish double chain silicates from single chain silicates. Below are the more common members of the inosilicates. See the Inosilicates' page for a more complete list.
Single Chain Inosilicates:
- Lorenzenite (Sodium Titanium Silicate)
- Neptunite (Potassium Sodium Lithium Iron Manganese Titanium Silicate)
- Okenite (Hydrated Calcium Silicate)
- Pectolite (Sodium Calcium Silicate Hydroxide)
- The Pyroxene Group:
- Aegirine (Sodium Iron Silicate)
- Augite (Calcium Sodium Magnesium Aluminum Iron Titanium Silicate)
- Diopside (Calcium Magnesium Silicate)
- Enstatite (Magnesium Silicate)
- Hedenbergite (Calcium Iron Silicate)
- Hypersthene (Magnesium Iron Silicate)
- Jadeite (Sodium Aluminum Iron Silicate)
- Spodumene (Lithium Aluminum Silicate)
- Rhodonite (Manganese Iron Magnesium Calcium Silicate)
- Serandite (Sodium Manganese Calcium Silicate Hydroxide)
- Shattuckite (Copper Silicate Hydroxide)
- Wollastonite (Calcium Silicate)
- The Amphibole Group:
- Actinolite (Calcium Magnesium Iron Silicate Hydroxide)
- Anthophyllite (Magnesium Iron Silicate Hydroxide)
- Cummingtonite (Iron Magnesium Silicate Hydroxide)
- Edenite (Sodium Calcium Magnesium Iron Aluminum Silicate Hydroxide)
- Hornblende (Calcium Sodium Magnesium Iron Aluminum Silicate Hydroxide)
- Riebeckite (Sodium Iron Silicate Hydroxide)
- Tremolite (Calcium Magnesium Iron Silicate Hydroxide)
- Astrophyllite (Potassium Iron Titanium Silicate Hydroxide)
- Babingtonite (Calcium Iron Manganese Silicate Hydroxide)
- Inesite (Hydrated Calcium Manganese Silicate Hydroxide)
Cyclosilicate Subclass (rings) 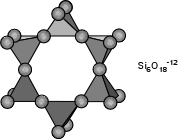
These silicates form chains such as in the inosilicates
except that the chains link back around on themselves to form rings. The
silicon to oxygen ratio is generally the same as the inosilicates,
(1:3). The rings can be made of the minimum three tetrahedrons forming
triangular rings (such as in benitoite). Four tetrahedrons can form a
rough square shape (such as in axinite). Six tetrahedons form hexagonal
shapes (such as in beryl, cordierite and the tourmalines). There are
even eight membered rings and more complicated ring structures. The
symmetry of the rings usually translates directly to the symmetry of
these minerals; at least in the less complex cyclosilicates. Benitoite's
ring is a triangle and the symmetry is trigonal or three-fold. Beryl's
rings form hexagons and its symmetry is hexagonal or six-fold. The
Tourmalines' six membered rings have alternating tetrahedrons pointing
up then down producing a trigonal as opposed to an hexagonal symmetry.
Axinite's almost total lack of symmetry is due to the complex
arrangement of its square rings, triangle shaped borate anions (BO3) and
the position of OH groups. Cordierite is pseudo-hexagonal and is
analogous to beryl's structure except that aluminums substitute for the
silicons in two of the six tetrahedrons. There are several gemstone
minerals represented in this group, a testament to the general high
hardness, luster and durability. Below are the more common members of
the cyclosilicates. See the Cyclosilicates' page for a more complete
list.- Axinite (Calcium Magnesium Iron Manganese Aluminum Borosilicate Hydroxide)
- Baratovite (Potassium Lithium Calcium Titanium Zirconium Silicate Fluoride)
- Benitoite (Barium Titanium Silicate)
- Beryl (Berylium Aluminum Silicate)
- Cordierite (Magnesium Aluminum Silicate)
- Dioptase [Copper Silicate Hydroxide]
- Eudialyte (Sodium Calcium Cesium Iron Manganese Zirconium Silicate Hydroxide Chloride)
- Milarite (Hydrated Potassium Calcium Aluminum Beryllium Silicate)
- Osumilite (Potassium Sodium Iron Magnesium Aluminum Silicate)
- The Tourmaline Group:
- Dravite (Sodium Magnesium Aluminum Boro-Silicate Hydroxide)
- Elbaite (Sodium Lithium Aluminum Boro-Silicate Hydroxide)
- Schorl (Sodium Iron Aluminum Boro-Silicate Hydroxide)
- Uvite (Calcium Sodium Iron Magnesium Aluminum Boro-Silicate Hydroxide)
- Sugilite (Potassium Sodium Lithium Iron Manganese Aluminum Silicate)
Phyllosilicate Subclass (sheets) 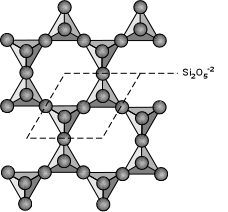
In this subclass, rings of tetrahedrons are linked by shared oxygens to
other rings in a two dimensional plane that produces a sheet-like
structure. The silicon to oxygen ratio is generally 1:2.5 (or 2:5) because
only one oxygen is exclusively bonded to the silicon and the other three
are half shared (1.5) to other silicons. The symmetry of the members of
this group is controlled chiefly by the symmetry of the rings but is
usually altered to a lower symmetry by other ions and other layers. The
typical crystal habit of this subclass is therefore flat, platy, book-like
and display good basal cleavage. Typically, the sheets are then connected
to each other by layers of cations. These cation layers are weakly bonded
and often have water molecules and other neutral atoms or molecules
trapped between the sheets. This explains why this subclass produces very
soft minerals such as talc, which is used in talcum powder. Some members
of this subclass have the sheets rolled into tubes that produce fibers as
in asbestos serpentine. Below are the more common members of the phyllosilicates. See the Phyllosilicates' page for a more complete list.
- Apophyllite (Hydrated Potassium Sodium Calcium Silicate Hydroxide Fluoride)
- Cavansite (Hydrated Calcium Vanadium Silicate)
- Chrysocolla (Hydrated Copper Aluminum Hydrogen Silicate Hydroxide)
- The Clay Group:
- The Chlorite Group:
- Chlorite (Iron Magnesium Aluminum Silicate Hydroxide)
- Clinochlore (Iron Magnesium Aluminum Silicate Hydroxide)
- Cookeite (Lithium Aluminum Silicate Hydroxide)
- Kaolinite (Aluminum Silicate Hydroxide)
- Pyrophyllite (Aluminum Silicate Hydroxide)
- Talc (Magnesium Silicate Hydroxide)
- Gyrolite [Hydrated Calcium Silicate hydroxide]
- The Mica Group:
- Biotite (Potassium Iron Magnesium Aluminum Silicate Hydroxide Fluoride)
- Lepidolite (Potassium Lithium Aluminum Silicate Hydroxide Fluoride)
- Muscovite (Potassium Aluminum Silicate Hydroxide Fluoride)
- Phlogopite (Potassium Magnesium Aluminum Silicate Hydroxide Fluoride)
- Zinnwaldite (Potassium Lithium Iron Aluminum Silicate Hydroxide Fluoride)
- Prehnite (Calcium Aluminum Silicate Hydroxide)
- Serpentine (Iron Magnesium Silicate Hydroxide)
The Tectosilicate Subclass (frameworks) 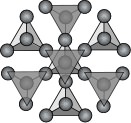
This subclass is often called the "Framework Silicates" because its
structure is composed of interconnected tetrahedrons going outward in all
directions forming an intricate framework analogous to the framework of a
large building. In this subclass all the oxygens are shared with other
tetrahedrons giving a silicon to oxygen ratio of 1:2. In the near pure
state of only silicon and oxygen the mineral is quartz (SiO2). But the
tectosilicates are not that simple. It turns out that the aluminum ion can
easily substitute for the silicon ion in the tetrahedrons up to 50%. In
other subclasses this substitution occurs to a more limited extent but in
the tectosilicates it is a major basis of the varying structures. While
the tetrahedron is nearly the same with an aluminum at its center, the
charge is now a negative five (-5) instead of the normal negative four
(-4). Since the charge in a crystal must be balanced, additional cations
are needed in the structure and this is the main reason for the great
variations within this subclass. Below are the more common members of the
tectosilicate subclass. See the tectosilicates' page for a more complete
list. - The Feldspar Group:
- Albite (Sodium Aluminum Silicate)
- Andesine (Sodium Calcium Aluminum Silicate)
- Anorthite (Calcium Aluminum Silicate)
- Bytownite (Calcium Sodium Aluminum Silicate)
- Labradorite (Sodium Calcium Aluminum Silicate)
- Microcline (Potassium Aluminum Silicate)
- Oligoclase (Sodium Calcium Silicate)
- Orthoclase (Potassium Aluminum Silicate)
- Sanidine (Potassium Aluminum Silicate)
- The Feldspathoid Group:
- Cancrinite (Sodium Calcium Aluminum Silicate Carbonate)
- Lazurite (Sodium Calcium Aluminum Silicate Sulfate Sulfide Chloride)
- Leucite (Potassium Aluminum Silicate)
- Nepheline (Sodium Potassium Aluminum Silicate)
- Sodalite (Sodium Aluminum Silicate Chloride)
- The Quartz Group: (All Silicon Dioxide)
- Coesite
- Cristobalite
- Quartz
- Tridymite
- Scapolite (Calcium Sodium Aluminum Silicate Chloride Carbonate Sulfate)
- The Zeolite Group:
- Analcime (Hydrated Sodium Aluminum Silicate)
- Chabazite (Hydrated Calcium Aluminum Silicate)
- Harmotome (Hydrated Barium Potassium Aluminum Silicate)
- Heulandite (Hydrated Sodium Calcium Aluminum Silicate)
- Laumontite (Hydrated Calcium Aluminum Silicate)
- Mesolite (Hydrated Sodium Calcium Aluminum Silicate)
- Natrolite (Hydrated Sodium Aluminum Silicate)
- Phillipsite (Hydrated Potassium Sodium Calcium Aluminum Silicate)
- Scolecite (Hydrated Calcium Aluminum Silicate)
- Stellerite (Hydrated Calcium Aluminum Silicate)
- Stilbite (Hydrated Sodium Calcium Aluminum Silicate)
- Thomsonite (Hydrated Sodium Calcium Aluminum Silicate)
Sulphates
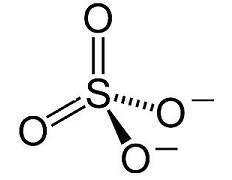
Sulphate minerals include the sulfate ion (SO42−) within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary minerals in the oxidizing zone of sulfide mineral deposits. Common examples include gypsum (CaSO4·2H2O) and anhydrite (CaSO4) in evaporitic sediments; barite (BaSO4), which is deposited from hydrothermal fluids; and anglesite (PbSO4), an alteration product of lead sulfide ores.
Sulphides
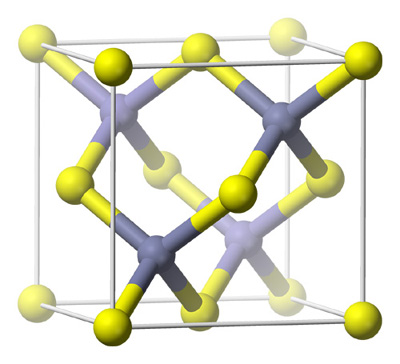
Sulphide minerals include the sulphide anion within their structure S-2
Common examples include the important metallic minerals: Sphalerite (Zinc Iron Sulfide), Galena (Lead Sulfide), Molybdenite (Molybdenum Sulfide), Pyrite (Iron Sulfide), Chalcopyrite (Copper Iron Sulfide)
The vast majority of sulfide minerals are components of hydrothermal sulfide ores. Some sulfides of Fe, Ni, Cu, and Pt are associated with processes of magma formation in ultrabasic rocks. Sulfide minerals may be of sedimentary origin, or they may be exogenous, having been deposited from surface solutions under the action of H2S—for example, in coal-bearing strata and in oxidation zones of sulfide deposits.
Non-metallic Minerals and their Properties
"click on a link to see an image of the mineral"N.B. most (if not all) minerals with colour listed as 'colourless' may
be tinted almost any colour by the presence of trace impurities.
| NAME FORMULA |
HABIT | LUSTRE S.g. |
USUAL COLOUR/ STREAK |
HARDNESS CLEAVAGE |
OTHER PROPERTIES |
OCCURRENCE USES |
|---|---|---|---|---|---|---|
| Calcite CaCO3 |
rhombohedral, dog tooth and nail head crystals |
vitreous 2.7 |
colourless or white white |
3 3 perfect, rhombohedral |
effervesces vigorously in dil. HCl. double refraction of images viewed throught it |
as limestone, in veins, as stalactites, stalagmites lime fertilizer, cement, flux in steel industry |
| Selenite gypsum CaSO42H2O |
lozenge crystals, often fishtail twins |
vitreous 2.3 |
colourless, white white |
2 1 perfect, parallel to crystal faces |
|
evaporites and free-growing in clays plaster-of -paris, plasterboard ie. Gyproc |
| Alabaster gypsum CaSO 42H2O |
crystalline, granular |
vitreous 2.3 |
colourless, white white |
2 n/a |
|
evaporites plaster-of -paris, plasterboard ie. Gyproc |
| Satin spar gypsum CaSO4 2H2O |
fibrous | vitreous 2.3 |
colourless, white white |
2 n/a |
|
evaporites plaster-of -paris, plasterboard i.e.. Gyproc |
| Halite (rock salt) NaCl |
cubic crystals, with hollow stepped faces |
vitreous 2.2 |
colourless, white white |
2 - 2.5 3 good, cubic |
tastes of salt, soluble in water (so clean it in petrol) |
evaporite, playa lake deposit food processing, washing soda |
| Quartz SiO2 |
elongated hexagonal prisms capped by pyramids, or crystalline granular |
vitreous 2.65 |
colourless in rock crystal - see guide to properties for other
varieties white |
7 none |
horizontal striations on crystal faces, conchoidal fracture |
as sand and sandstone, in veins, as geodes, constituent in many igneous and metamorphic rocks microchips, gemstone. concrete and glass making (as sand) |
| Barite BaSO4 |
tabular crystals, reticulate crystals, or radiating bladed 'cockscomb' structures in crystalline form |
vitreous 4.5 |
colourless, white white |
2.5 - 3.5 3 good, 1 horizontal (basal), 2 vertical (at 90
degrees to basal) |
heaviest vitreous mineral at s.g.4.5 |
vein mineral used in paint and paper, and as a drilling mud in the oil industry |
| Biotite mica K2(Mg,Fe2+) 6-4(Fe3+ Al,Ti)0- 2[Si6-5Al2 -3O20] OH,F)4 |
foliaceous, platey, pseudo- hexagonal crystals |
pearly 3.0 |
brown, black white |
2.5 - 3 1 perfect basal |
|
a common rock forming mineral in sedimentary, metamorphic and igneous rocks used for furnace windows heat resistant formica and as glitter in a wide range of products. |
| Muscovite mica K2Al4 [Si6Al2O20] (OH,F)4 |
foliaceous, platey, pseudo- hexagonal crystals |
pearly 3.0 |
silvery-white (named after Moscow in 'White Russia' white |
2 - 2.5 1 perfect basal |
|
a common rock forming mineral in sedimentary, metamorphic and igneous rocks used for furnace windows heat resistant formica and as glitter in a wide range of products. |
| Fluorite, Blue John CaF2 |
cubic crystals or as crystalline masses |
vitreous, sometimes a very slightly greasy or watery appearance
2.7 |
colourless, blue & yellow as blue john variety white |
4 4 perfect octohedral:- cleavage planes cut across
corners of 6-sided cubic crystals to leave 8-sided octohedral
cores. |
blue john is coloured by oil impurities and is only found in situ at Mam Tor in Derbyshire, UK |
vein mineral semi- precious gemstone as blue john (from French; bleu-blue jaune-yellow). Also used as flux in steel smelting |
| Orthoclase feldspar (complex framework silicate) |
prismatic, tabular or rectangular crystals |
vitreous to pearly about 2.6 |
colourless (may be cloudy), white, pink or pale red, other pale
colours also white |
6 (a moh's scale mineral) 2 cleavages at 90 degrees |
sub- transparant to translucent orthoclase is named after it's 2 cleavages at right angles: ortho=right klastos =breaks, |
common rock forming mineral in igneous, metamorphic and sedimentary rocks used in vitreous chinaware and as an abraisive in scouring powders. |
| Plagioclase feldspar (complex framework silicate) |
prismatic, tabular or rectangular crystals |
vitreous to pearly about 2.7 |
white or grey to grey-blue or other pale colours white |
6 - 6.5 2 cleavages at almost 90 degrees |
sub- transparant to translucent plagioclase is named after its 2 cleavages at almost right angles: plagio=almost klastos=breaks |
common rock forming mineral in igneous, metamorphic and sedimentary rocks used in vitreous chinaware and as an abraisive in scouring powders. |
| Oligoclase feldspar (complex framework silicate) |
prismatic, tabular or rectangular crystals |
vitreous to pearly about 2.6 |
white or pale colours white |
2 cleavages at almost 90 degrees 6 - 6.5 |
sub- transparant to translucent oligoclase is named after it's cleavages at a few degrees from a right angle: oligo=a few klastos =breaks |
common rock forming mineral in igneous, metamorphic and sedimentary rocks used in vitreous chinaware and as an abraisive in scouring powders. |
| Olivine FeMgSiO4 |
tabular crystals or granular crystalline masses |
vitreous 2.5 - 3.5 (more often ~3.5) |
green, may also be yellow or brown white |
1 poor cleavage, cracks on what appears to be second cleavage
plane; actually a sub-parallel fracture 6 - 7 |
|
occurs in basic and ultrabasic igneous rock , best crystals occur in olivine-peridotite gemstone |
| Garnet (a group of Fe,Ca,Al,Cr, Mn & Mg, silicate minerals) |
rhombo- dodecahedral, dodecahedral and tetra- hexahedral crystals, also as angular fragments |
vitreous 3.6 - 4.3 |
deep red, crimson, purple, brown, black, olive, greens, pink,
yellow white |
no cleavage 7 - 7.5 (except gossular variety, which may be as
low as 6.5) |
|
a dense mineral formed in high pressure /temperature condition in metamorphic rocks used as a gemstone and as an abraisive (garnet paper is a red abraisive paper used on wood). |
| Malachite Cu2[(OH) 2CO3] |
crypto- crystalline |
dull 4.0 |
emerald green, or other shades of green light green, |
good 4.0 |
|
a weathering product of copper deposits |
| Chrysocolla Cu4H4 [(OH)8Si4 O10] |
crypto- crystalline |
dull 2.0 - 2.3 |
blue-green pale green or blue-green |
none 2 - 4 |
|
in oxidation zones in copper ore deposits |
Metallic Minerals and their Properties
"click link to see an image of the mineral"| NAME FORMULA |
HABIT | LUSTRE S.g. |
USUAL COLOUR/ STREAK |
HARDNESS CLEAVAGE |
OTHER PROPERTIES |
OCCURRENCE USES |
|---|---|---|---|---|---|---|
| Galena PbS |
cubic crystals | metallic 7.5 |
silver-grey grey-black |
2.5 3; perfect cubic cleavage |
|
vein mineral, often with calcite, fluorspar and barites cheif
source of lead (Pb). |
| Haematite / Hematite Fe2O3 |
reniform aggregate habit | earthy 5 |
Red-brown red-brown |
5.5 to 6.5 2 poor cleavages |
sub-conchoidal or uneven fracture | often in limestones as a relacement minerals, also in
metamorfphic deposites, ironstones and as both thin veins and
cement in sandstones eg. the New Red Sandstone iron ore and used
as a pigment in paint 'Red Ochre' |
| Pyrite FeS2 |
cubic crystals, or as pentagonal dodecahedral crystals. Also as nodules with an internal structure of radiating needles, also as crystalline masses | metallic 5.0 |
brass-yellow black |
6 - 6.5 no cleavage |
cubic crystals often have striated faces, conchoidal fracture, sparks when struck with geological hammer pyrite = fire mineral. smells sulphurous when rubbed on a streak plate |
occurs as free crystals or nodules in coal, clay and shales,
also in veins formerly source of sulphur, used to make sulphuric
acid (native sulphur now main source). |
| Sphalerite (aka zinc blende, black
jack) ZnS |
usually massive aggregates, also tetrahedral crystals | resinous, sometimes brilliant or adamantine on fresh surfaces
4.0 |
brown or black pale yellow |
3.5 - 4 perfect cleavage in 6 directions |
smells sulphurous when rubbed on a streak plate | vein mineral chief source of [non-corrosive] zinc, used for
galvanisation of iron |
| Chalcopyrite CuFeS2 |
octohedral and tetrahedral crystals | metallic 4.1 - 4.3 |
brass-yellow black or greenish-black |
3.5 - 4.0 indistinct |
|
ore mineral |
List of common minerals and their images
'click on any name to see an image..."anhydrite apatite 1 2 arsenopyrite asbestos barite calcite 1 2 cassiterite chalcopyrite 1 2 chlorite chromite corundum diamond feldspar-albite feldspar-microcline feldspar-orthoclase 1 2 3 4 feldspar-plagioclase 1 2 3 fluorite1 2 galena 1 2 garnet gold graphite gypsum 1 2 halite(salt) hematite/haematite hornblende 1 2 ilmenite kyanite limonite magnetite 1 2 manganese ore mica-biotite1 2 mica-muscovite1 2 3 mica-phlogopite molybdenite olivine pyrite1 2 pyroxene1 2 pyrrhotite quartz1 2 3 4 5 6 rutile siderite smaltite sphalerite1 2 stibnite talc1 2 3 topaz1 2 tourmaline zircon

