Exploding Lakes -limnic eruptions
Exploding Lakes - limnic eruptions

Lake Kivu - holds the potential form catastrophic explosion
Basic Ingredients for an Exploding Lake
Examples and Variants of Explosive Lakes
How to Reduce the Risk of Limnic Explosion
Methane extraction from Lake Kivu
see
also the potential explosive de-gassing of methane hydrates...
Sources
.Basic Ingredients for an Exploding Lake
Carbon dioxide CO2 is a natural by-product of geologic processes. Limnic lakes are capable of explosively producing large volumes of carbon dioxide CO2
Since the gas is heavier than air, it will accumulate in depressions and low lying areas causing asfixiation and death.CO2 poisonings have occurred in nature. Since Roman times, vented carbon dioxide in volcanic central Italy occasionally has killed animals or people who have wandered into topographic depressions where the heavier than air gas pools. At Yellowstone National Park, grizzly bears have met the same fate in a ravine known as Death Gulch.
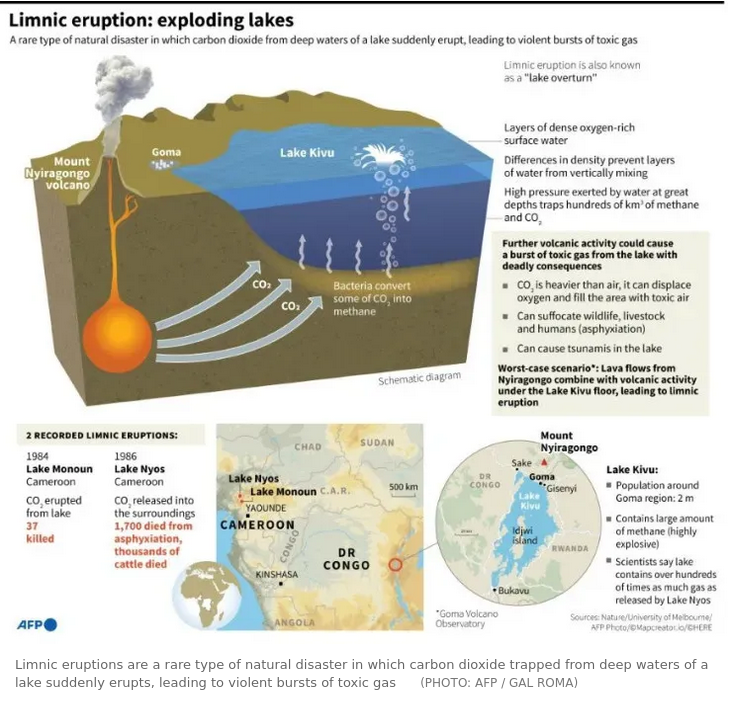
suggested conditions for limnic eruptions
- a very deep very still lake
- the water stratified by temperature and/ or density into two or more layers
- a volcanic vent (likely a diatreme) or rift at the bottom of the lake
- carbon dioxide rich magma venting bubbles of CO2 carbon dioxide gas into the deep still lake water
- flammable hydrogen sulphide H2S may also be vented and absorbed in the water and added to the saturated gas load
- algae may also sink into the depths decay and produce flammable methane CH4 adding to the saturated gas load
- the CO2 carbon dioxide dissolves into the deep lake water until it forms an extremely saturated layer at depth hence under pressure
- a geological or other event disturbs the saturated layer pushing some of it upward
- the dense saturated layer moving upwards experiences lower pressure and the CO2 carbon dioxide comes out of solution - the bubbles move towards the surface
- the rising bubbles drag more dense saturated water upwards --> more bubbles--> --> more water dragged upwards --> repeat --> repeat--> an ever increasing chain of events creates an enormous explosive gas release
- danger is increased if the released gas is flammable
- this is referred to as a limnic explosion

Water stratification and methane concentrations in Lake Kivu
Examples and Variants of Explosive Lakes
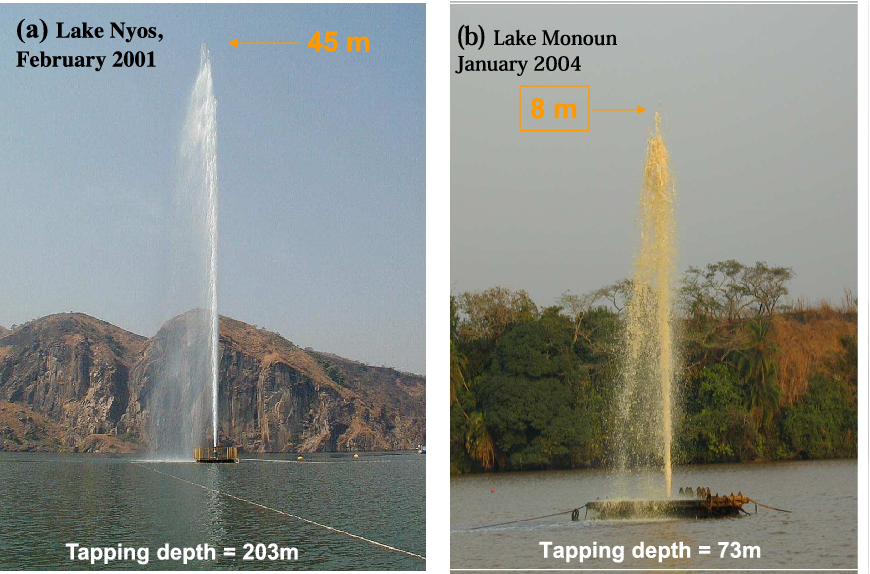
- Lake Monoun in Cameroon exploded in 1984, causing
asphyxiation and death of 37 people living nearby.
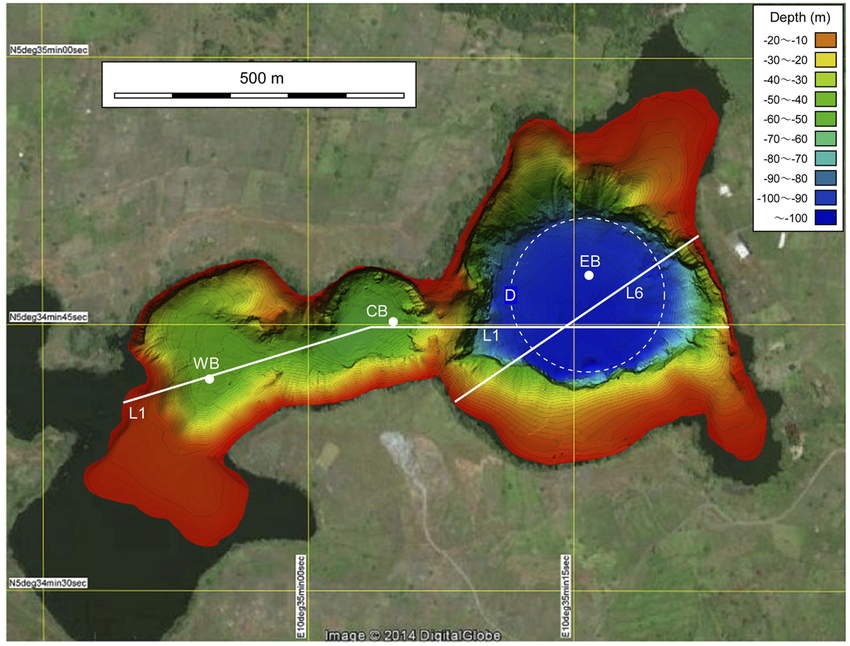
possible diatreme (gas vent - blue) under Lake Monoun
- Lake Nyos in Cameroon - exploded in 1986, releasing over 80
million m3 of CO2
Nyos is a crater lake, a chasm blasted out of the land by volcanic activity around 400 years ago. Something disturbed the lake - an eruption, a landslide and the accumulation of cold rainwater on the surface... This forced the less dense upper layer of the lake to sink while the denser, deeper layer of the lake rose. The result was catastrophic. The lake experienced severe turbulence, sufficient enough to allow its deep pockets of dissolved carbon dioxide to bubble out of solution en masse. In just 20 seconds, 1.25 km3 (0.3 cubic miles) of the phantom gas gushed out of the lake and being heavier than air, tumbled downslope, managing to reach villages as far as 25 kilometres ( 16 miles) away. Killing 1,746 people by asphyxiation, along with 3000 livestock animals.

- Lake Pavin in France, Lake Albano in Italy, Lake Monticchio in Italy - even though these lakes are in temperate climates that favor seasonal overturning of lake waters and (thus gradual release of accumulated gas). There is still no agreement on the dynamics and causes of eruptions but they appear to show markers of limnic eruptions such as
- reworked sediments with reversed ages,
- brown colors of sedimentary deposits,
- gas-rich sediments,
- iron hydroxide-rich sediments,
- strong Ti and Fe enrichments,
- sedimentary hiatuses,
- absence of seismic evidence in the sedimentary record, and
- significant change in geochemical signature
- Lake Kivu on the border of Rwanda and the Democratic Republic of
the Congo - a ticking timebomb
- Sediment samples taken from the lake showed a limnic event cause living creatures in and around the lake to go extinct around about every 1,000 years, causing vast amounts vegetation to be swept back into the lake where it decays to produce methane.
- So both carbon dioxide and methane are a concern as is the proximity of the large city of Goma population: 800,000
- The lake is probably the world's largest natural freshwater digester of algae to produce methane biogas.
- Extracting methane gas is critical to avert a future limnic eruption. As a benefit methane can be used to generate electricity
- Undisturbed and stored in the lower strata of the lake, the reservoir formed by salinity-based chemoclines, keeps biogenic CH4 and CO₂ in solution.
- the lake contains at least 291km3 (70 cubic miles) of CO2 carbon dioxide and 62.5km3 (15 cubic miles) of flammable CH4 methane, that’s produced by the exhalations of microbes that eat a small amount of the plentiful carbon dioxide. Methane isn’t just toxic; it’s also highly combustible. Pockets of it gathering on land could ignite and explode. If even a fraction of this gas is unleashed on Kivu’s urbanised shorelines, it would kill thousands. . Scientists are acutely aware of this hazard. For now, the gases are trapped beneath an aquatic lid: the weight of 457 metres (1,500 feet) of water above, and an additional dense layer of salty water, keeps them from rushing to the surface.
- lava lake of Mount Nyiragongo in the Democratic Republic of
the Congo
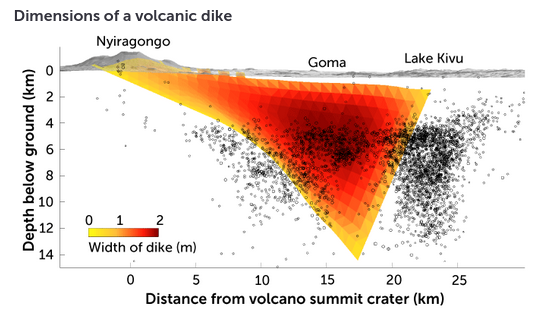
- Carbon dioxide is a volcanic gas eructed by many volcanoes around the world, but Mount Nyiragongo is something else entirely: it is one of the most prolific natural emitters of carbon dioxide on the planet.
- At about 6:30 p.m. (CAT) on 22 May 2021, the lava lake filled the Mount Nyiragongo crater, the crater wall was breached and lava began to rapidly flow towards the city of Goma resulting in 32 deaths, 3,629 homes and buildings destroyed, more than 20,000 displaced
- Having a high CO2 gas content, the lava lake bubbles constantly and the lava is low viscosity (free-flowing) hence fast moving
- The magma beneath the volcanoe migrated under the city of Goma and Lake Kivu (see image above)
- Notable eruptions have occured throughout the 20th and 21st centuries.in 1977 and again in 2002 when it displaced about 400,000 people when the lava lake in the summit crater drained catastrophically, causing a massive lava flow that reached the city of Goma and claimed several lives
How to Reduce the Risk of Limnic Explosion
The researchers considered various measures such as...- Blowing out the carbon dioxide by dropping bombs (too dangerous);
- Dumping in massive quantities of lime in order to neutralize the gas (too expensive);
- Digging tunnels in the lake bed to drain the gas-laden bottom waters (way too expensive).
- Running a pipe from the lake's deepest water layer to the surface turns out to be the best choice - , gradually releasing the gas to disperse quickly and harmlessly in the air. In theory, such a pipe, once primed, would carry the pressurized water from the depths and shoot it into the air like a natural geyser—a controlled explosion that could be sustained for years.
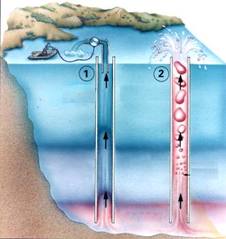
de-gassing the lake by running pipes to the saturated layer - 1. by pumping 2. by natural pressure release
Fountains produced by de-gassing - Gas rises on its own as pressure is reduced
Of course the de-gassing is an on-going process, as new gas is continuously vented into the lake from below.
For Lake Nyos de-gassing is proving to be insufficient: carbon dioxide is still building up in the lake faster than the pipes can release it.
Methane extraction from Lake Kivu
Lake Kivu due to the rotting of vast amount of vegetation has significant and economic levels of methane CH4 in it's stratification layers that are driven not only by dissolved gas but also by dissolved salt HCl.Extracting gas is essential to avert a future limnic eruption. Undisturbed, the reservoir formed by salinity-based chemoclines, keeps biogenic CH4 and CO₂ in solution. This is stored in lower salty chemocline layer deep in the lake. Gases, ever accumulating closer to saturation levels, threaten to cause a future limnic eruption much bigger than experienced before - Lake Kivu has potential and inventory for consequences 1000 times larger,The key to safety is management of the chemoclines while producing payable gas. to cover the cost of making the lake safe.

Rwanda makes a small profit from the methane , raw methane gas is extracted by washing with water to make the gas fit for use in power-generation and domestic gas. However the remaining toxic gases CO2 and H2S are left behind. The challenge lies in how to minimise the negative toxic effects of H₂S, from CO₂-induced acidity, and oxygen depletion by CH4 and H₂S.
Natural upwelling of saline meteoric water from lava strata into the deep nutrient-rich water bodies must be avoided as it has the potential to initiate a limnic explosive chain event.
The extracted cold "deep water "may present additional hazards. The process might set off a new explosion by spurting cold, dense bottom water onto the surface of the lake; the water would sink and create turbulence below.
This facility won’t, however, have a significant mitigating effect on the Lake Kivu's hazards.
The extraction rig is small and the lake’s carbon dioxide is another matter entirely—it’s essentially unaffected by this gas extraction scheme.
It is yet unknown how these changes in the chemical balance will affect Lake Kivu in the future.
Sources
https://sci-hub.ru/10.1016/j.jafrearsci.2019.103672https://sci-hub.ru/10.1029/2010eo300008
https://sci-hub.ru/10.2307/40175169
https://sci-hub.ru/10.1016/j.jafrearsci.2019.10367
https://www.nature.com/immersive/d41586-021-02523-5/index.html
https://en.wikipedia.org/wiki/Limnic_eruption
https://limniceruptions.weebly.com/
https://www.ibtimes.com/dr-congo-eruption-false-alarm-humanitarian-crisis-mounts-3212081
https://www.smithsonianmag.com/science-nature/defusing-africas-killer-lakes-88765263/
https://www.forbes.com/sites/robinandrews/2021/05/31/nyiragongo-limnic-eruptions-explained/
https://readtheimpact.com/lake-kivu-the-danger-of-a-limnic-eruption/
https://en.wikipedia.org/wiki/Limnic_eruption
https://www.semanticscholar.org/paper/Lakes-Nyos-and-Monoun-Gas-Disasters-Eruptions-by-of-Kusakabe/1a1fb5a11e97fb9c1b103199fd832d4f5d2801de/figure/9
https://www.lakepedia.com/lake/kivu.html
https://www.researchgate.net/publication/336606923_Managing_the_dangers_in_Lake_Kivu_-_How_and_why
https://www.sciencedirect.com/science/article/pii/S0012825223002921

