Methane hydrates primer
Methane hydrates primer
What are Methane hydrates and why are they
important?
How do Methane Hydrates form?
Affect on climate of a release of methane
Methane Hydrates and Past Warming Events
Fate of Contemporary Methane Hydrates During Warming
Climate
Global Warming and Gas Hydrate Type Locales
Conclusions
see
also Exploding lakes - limnic explosions...
Sources
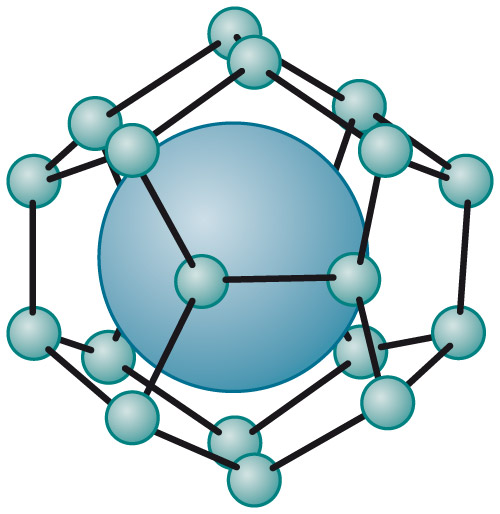
a methane molecule in a "water cage" is a methane hydrate
What are Methane hydrates and why are they important?
Gas hydrates are ice-like crystalline structures that form in deep-sea sediments when a low-density gas, like methane (CH4), ethane (C2H6), or carbon dioxide (CO2), combines but does not chemically bond with water and freezes into a solid under low temperature and moderate pressure conditions.
Various names include methane clathrate, hydromethane, methane ice, fire ice, natural gas hydrate, or gas hydrate.
While, for reasons unknown, often sidelined in climate remediation strategy discussion, their relatively rapid release into an already warming atmosphere need to be taken into account. Consequences to ocean life, continental life, humankind, ocean acidification and adding greenhouse gas to the atmosphere. There are plans, in some circles, to exploit methane hydrates as a fossil fuel resource with the risk of accelrated release of Methane CH4

methane "ice" in permafrost
On Earth, gas hydrates occur naturally in some marine sediments and within and beneath permafrost. They are likely found on other planets.
The Importance of Methane Hydrate:
- Huge Carbon Sink - maybe twice the carbon contained in all reserves of coal, oil, and conventional natural gas combined,
- Potentially huge fossil fuel source but it comes with risk...
- Huge Environmental Risk - Their decomposition could release large amounts of the greenhouse gas methane, that will negatively impact Earth’s climate.
- Potential Explosive Release - The sudden release of pressurized methane gas could cause huge submarine landslides, which in turn could trigger tsunamis.
- Biological risks - Gas hydrates in the ocean associated with unusual and possibly unique biological communities that use hydrocarbons or hydrogen sulfide for carbon and energy, via a process known as chemosynthesis. We do not know the extent or significance of these process or what role they play in nature.
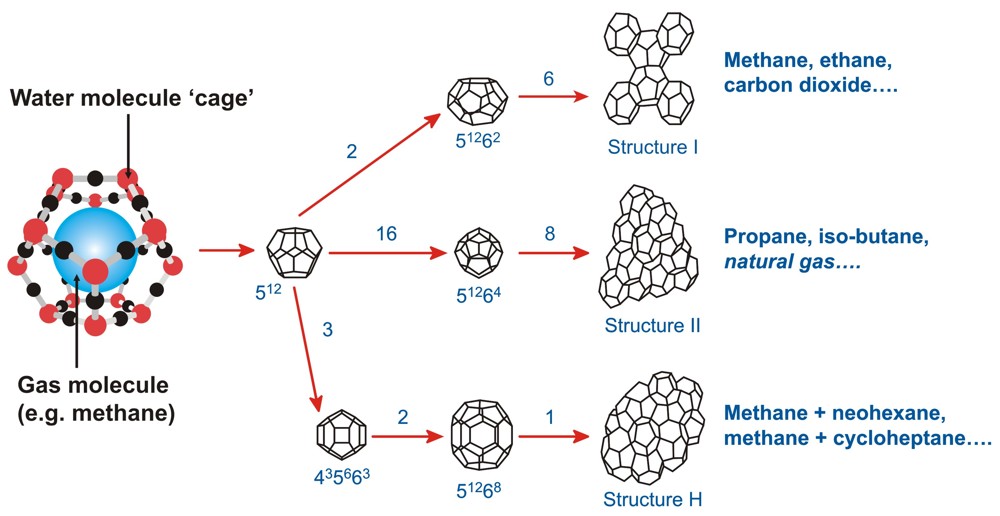
Methane in a "water cage" and the types of "ice" structures it can form
How do Methane Hydrates form?
Methane hydrate is an ice-like substance formed when CH4 and water combine at low temperature (up to about 25ºC) and moderate pressure (greater than 3-5 MPa, which corresponds to combined water and sediment depths of 300 to 500 m). Globally, an estimated 99% of gas hydrates occurs in the sediments of marine continental margins. Methane hydrate concentrates methane CH4 by about 164 times on a volumetric basis compared to gas at standard pressure and temperature.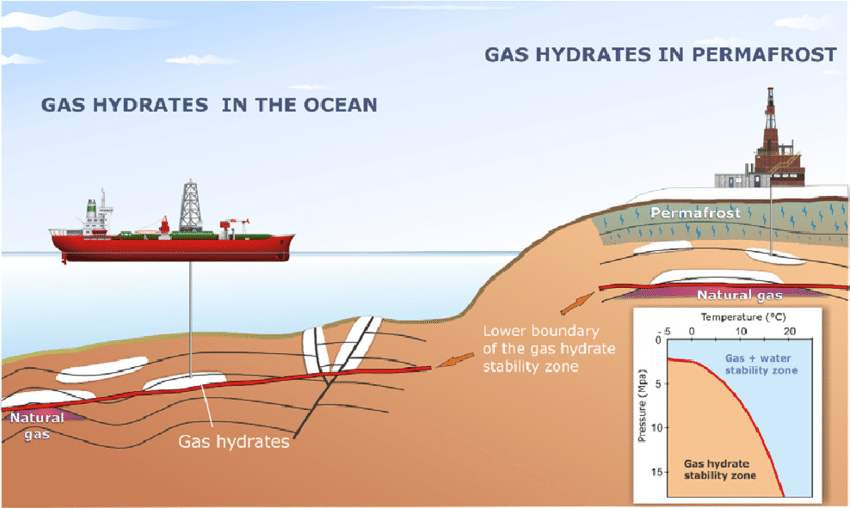
In the oceans - methane hydrates occur mainly near the continental margins at water depths between 350 and 5000 metres. Here methane gas is primarily formed by microorganisms that live in the sediment layers and slowly convert organic substances to methane. Methane can be released by a drop in pressure and/or a rise in temperature.
These organic materials are the remains of plankton that lived in the ocean long ago, then sank to the ocean floor, and were finally incorporated into the sediments.
The sea floor is thus an ideal location for methane hydrate formation: because...
- the bottom waters of the oceans and the deep seabed are almost uniformly cold, with temperatures from 0 to 4 degrees Celsius.
- Below a water depth of about 350 metres, the pressure is sufficient to stabilize the hydrates.
Almost no methane hydrate is found in really deep water. While the temperature and pressure are OK, the water is nutrient poor and produces insufficient organic matter for methane production/ concentration.
In the Arctic - permafrost, is mostly a relic of the last ice age. While the top layer of permafrost may melt in summer most of it remains frozen and the top layer re-freezes each year. Called the "active layer" it thaws during the summer, allowing plants to grow in a variety of ecosystems—such as grasslands, forests, and wetlands. In some parts of the Arctic, the landscape features a polygon-like pattern, formed when the frozen ground contracts evenly is all directions, forming cracks during the cold winter months. These cracks fill with meltwater in spring, which then freezes, creating ice wedges and giving the surface its distinct, appearance. Permafrost is predominantly found in the Northern Hemisphere, particularly in the Arctic region, where it covers about a quarter of the land’s surface. In places like Siberia, it can reach depths of up to 1500 meters. As atmospheric temperatures rise, the "active layer" is getting thicker in summer and facilitating the release of methane gas CH4 from the ancient frozen permafrost methane hydrates.
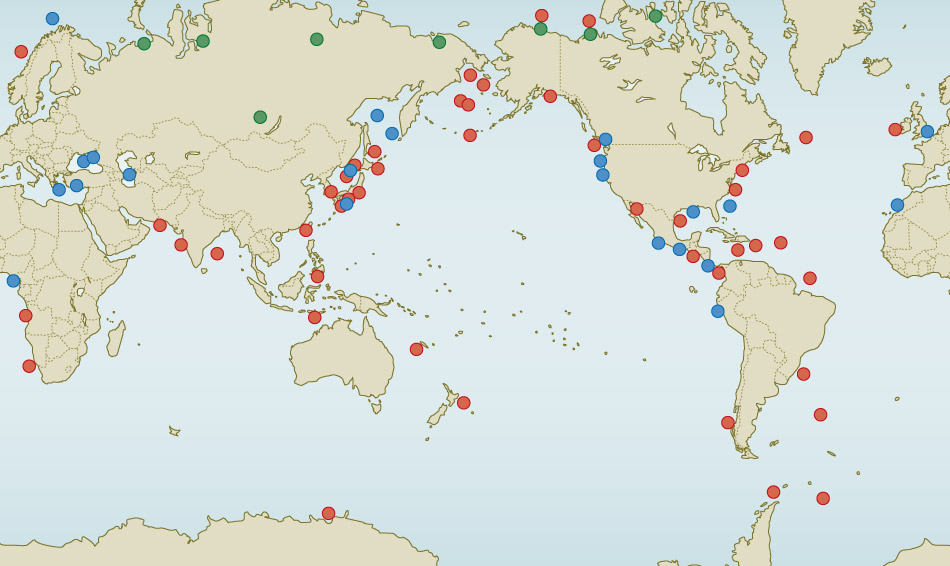
global locations of methane hydrate
Commercial extraction of methane hydrates in the deep ocean would involve putting a dual-tube drill string (pipe) into a large concentrated methane hydrate deposit and
either applying heat or reducing pressure. The methane would sublimate into gas and come to the surface under its own pressure.
There are problems to this however, once the gas begins to rise, the pressure will reduce at the deposit end of the drill string, releasing more gas.
So more surface area of the deposit is exposed increasing the rate of sublimation...
- A self-sustaining accelerating process could start resulting in explosive release of methane.
- If the drill string remained intact the gas could explosively release to the atmosphere.
- If the drill string was ruptured much of the gas would be re-absorbed in the ocean water creating a "pocket" of acidified water , killing marine life.
- How long would it this "pocket" would take to dissipate in the still waters of the deep ocean?
- When drilling through loose sediment into "ice" - how do you secure the wellhead to an ocean floor, to prevent leakage or collapse, when it consists of loose sediment and "ice"?
Affect on climate of a release of methane
The release of increased methane into the atmosphere , whether slowly or catastrophically is bad news for us all.- If just 0.1% (1.8 Gt C) of this CH4 were instantaneously released to the atmosphere, CH4 concentrations would, it has been estimated would increase from about 1774ppb (2005 value) to around 2900 ppb (source IPCC 2007).
- Methane CH4 is about 20 times more potent than CO2 as a green house gas..
- Methane CH4 concentrations have already risen by about 150% since pre-industrial times, compared to only about 40% for carbon dioxide CO2
- It oxidizes carbon dioxide CO2 after about a decade in the atmosphere.
- In recent models, the longer-lived CO2 oxidation product , not the CH4 itself, is credited with causing most of the excess atmospheric warming but that could change that would follow large-scale dissociation of methane hydrates.
- Rising atmospheric CH4 concentrations lead to more rapid depletion of the hydroxyl radicals needed for oxidation, this would mean longer CH4 residence times, and thus increased CH4-induced warming
- Present-day CH4 emissions are dominated by wetlands, ruminants, fossil fuel production, and rice cultivation sources that fluctuate with season, human behavior, and other factors.This makes detection of hydrate derived methane from melting release or commercial extraction difficult to separate from the background variations
Methane Hydrates and Past Warming Events
The geologic record is punctuated by warming events, measurements of the methane content in ice cores, may provide clues about future interactions between methane hydrates and contemporary climate change.
Climatic changes in the past could resulted from destabilization of methane hydrates and thus to the release of methane.
For example:
- The Paleocene-Eocene Thermal Maximum (PETM) at ~54.95 Ma where a large, negative carbon isotopic excursion (CIE) recorded in both marine and terrestrial sediments has been interpreted as reflecting widespread release of isotopically-light (microbial) carbon from dissociating marine methane hydrates
- Repeated warming of intermediate ocean waters during the Late Quaternary (since 400 ka) triggered periodic hydrate dissociation events
- Northern hemisphere wetlands, which may experience increased production of isotopically-light CH4 in response to local warming, appear to be the key culprit in enhancing atmospheric CH4 concentrations during several Pleistocene (~2.6 Ma to 10 ka) and Holocene (since 10 ka) warming events.
Fate of Contemporary Methane Hydrates During Warming Climate
The susceptibility of gas hydrates to warming climate depends on:
- the duration of the warming event,
- their depth beneath the seafloor or tundra surface, and
- the amount of warming required to heat sediments to the point of dissociating gas hydrates.warming events
Even over 100 yr, only gas hydrates close to the seafloor and initially within a few degrees of the thermodynamic stability boundary might experience dissociation in response to reasonable rates of warming.
Less than 5% of the gas hydrate inventory may meet these criteria.
Global Warming and Gas Hydrate Type Locales
Methane hydrates occur in five geographic locales that must be individually evaluated to determine their susceptibility to warming climate (see Figure below).
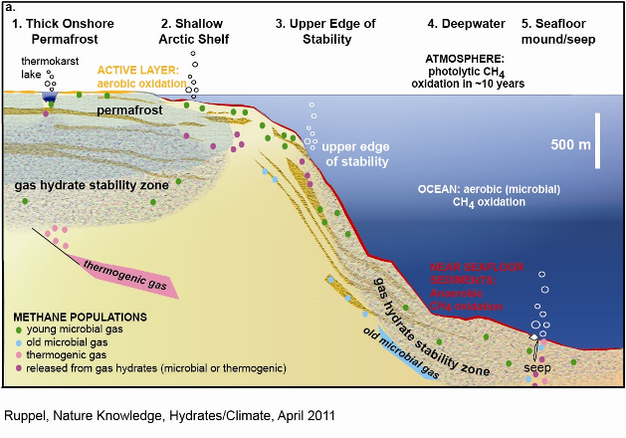
The 5 methane hydrate locales described below:
GHSZ below refers to the gas hydrate stability zone in the diagram
Locale 1. Thick (> 300 m) continuous permafrost onshore (<1%).
Deep gas hydrates beneath capping, permafrost-bearing sediments are stable over warm periods that endure more than 100 yr , even under scenarios of doubling atmospheric CO2
Only gas hydrates to a depth of about 225 m depth for pure CH4 hydrate within permafrost, might be vulnerable to dissociation due to atmospheric warming over 100 yr.
Locale 2. Subsea permafrost on the circum-Arctic shelves (<0.25%?).
Sediments on shallow marine continental shelves that fringe the Arctic Ocean are often underlain by permafrost and associated gas hydrates that formed in Pleistocene time, when these regions were sub-aerial and exposed to much colder annual temperatures.
Since the Late Pleistocene, marine inundation of these former coastal plains has led to large (up to 17ºC) temperature increases, partial thawing of sub-sea permafrost and inferred dissociation of gas hydrates.
Locale 3. Deepwater marine hydrates at the feather edge of Gas Hydrate Stability Zone GHSZ (~3.5%).
The deepwater marine hydrate system thins to vanishing at shallow water depths (usually about 500 m) on the upper continental slopes. Because the entire GHSZ lies near the seafloor, upper continental slopes are the most susceptible places on Earth for wholesale gas hydrate dissociation driven by warming of impinging intermediate ocean waters. A maximum 3.5% of the global gas hydrate inventory might occur in these vulnerable settings.
Locale 4. Deepwater gas hydrates (~95.5%). These gas hydrates, which constitute most of the global inventory, generally have low susceptibility to warming climate over time scales shorter than a millennium. The gas hydrates closest to the edge of thermodynamic stability lie deep within the sedimentary section and close to the base of the GHSZ. Sustained bottom water temperature increases lasting many 100 yr would be required to initiate warming, no less dissociation. Even if CH4 is released from gas hydrate and is able to migrate toward the seafloor, some CH4 may be trapped in newly formed gas hydrate and much will be consumed on the sediment/ocean boundary.
Locale 5. Seafloor gas hydrate mounds (trace).
At some marine seeps, massive, relatively pure gas hydrate occurs in seafloor mounds (e.g., Gulf of Mexico) and in shallow subsea floor layers or conduits. These mounds are shown schematically as deepwater phenomena in the above Figure, but in fact often occur at upper continental slope depths. While seafloor gas hydrate mounds and shallow sub-sea floor gas hydrates constitute only a trace component of the global gas hydrate inventory, they can dissociate rapidly due to expulsion of warm fluids from the seafloor, warming of overlying waters , or possibly pressure perturbations .
Conclusions
- Natural catastrophic, widespread dissociation of methane gas hydrates will not be triggered by continued climate warming at contemporary rates (of about 0.2ºC per decade) over timescales of a few hundred years.
- Most methane hydrates occur at low saturations and in sediments at such great depths below the seafloor or onshore permafrost that they will barely be affected by warming over 100 yr.
- Even when CH4 is liberated from gas hydrates, oxidative and physical processes may greatly reduce the amount that reaches the atmosphere as CH4.
- The CO2 produced by oxidation of CH4 released from dissociating gas hydrates will likely have a greater impact on ocean acidification and greenhouse gas levels.
- Contemporary and future gas hydrate degradation will occur primarily on the circum-Arctic Ocean continental shelves
- and at the feather edge of the GHSZ on upper continental slopes (Locale 3), where the zone's full thickness can dissociate rapidly due to modest warming of intermediate waters.
What happens when methane hydrate melts?
- Not all the methane that is released from unstable methane hydrates ends up in the atmosphere. The greatest portion is likely to be broken down during its rise through the sediments and in the water column. This decomposition is mediated by two biological processes: anaerobic oxidation of methane by bacteria and archaea within the sea floor; aerobic oxidation of methane by bacteria in the water column.
- During anaerobic oxidation of methane in the sediment the microbes use sulphate (SO42–), for the methane decomposition. At the end of this process calcium carbonate (CaCO3) precipitates, which can remain stored in the sea floor .
- During aerobic oxidation in the water column, bacteria break down methane with the help of oxygen (O2). This process has two negative effects
- carbon dioxide is produced and contributes to ocean acidification and
- aerobic oxidation of methane consumes oxygen. create or expand fish / marine life threatening low oxygen zones
- Rough estimates suggest that anaerobic and aerobic oxidation of methane together currently convert around 90 per cent of the methane produced in the sea floor before it can reach the atmosphere. The more slowly methane migrates through the sea floor or through the water column, the more effective the microbes are in converting it. A prerequisite for this kind of degradation is that the methane molecules are dissolved in water. Methane can only be degraded by the bacteria in this form.
The disappearance of methane hydrates could have fatal consequences. Gas hydrates act like a cement that fills the pores between the fine sediment particles and stabilizes the sea floor. If the methane hydrates decompose, the stability of the sea floor is reduced due to the missing cement and the possible generation of excess pore pressure. In the worst case, large parts of continental margins fail. The resulting submarine landslides might cause severe tsunamis. The question is - do we really need to exploit this resource or would it be more prudent to leave it alone?
Sources
https://www.researchgate.net/figure/llustration-of-methane-hydrates-recovery-onshore-from-below-the-permafrost-in-the-Arctic_fig1_308972926
https://oceanexplorer.noaa.gov/facts/hydrates.html
https://hydrate.site.hw.ac.uk/what-are-gas-hydrates/
https://www.sciencenewstoday.org/permafrost-the-sleeping-giant-of-climate-change
https://www.cbc.ca/news/science/methane-hydrates-energy-s-most-dangerous-game-1.701176
https://worldoceanreview.com/en/wor-1/energy/methane-hydrates/
https://worldoceanreview.com/en/wor-1/ocean-chemistry/climate-change-and-methane-hydrates/
https://en.wikipedia.org/wiki/Methane_clathrate
https://www.sciencedirect.com/topics/earth-and-planetary-sciences/methane-hydrate
https://www.nature.com/scitable/knowledge/library/methane-hydrates-and-contemporary-climate-change-24314790/
image: Ruppel, C. et al. Degradation of subsea permafrost and associated gas hydrates offshore of Alaska in response to climate change. Sound Waves 128, 1-3 (2010). http://soundwaves.usgs.gov/2010/11

