Hydrothermal Vein Deposits
Hydrothermal Vein Deposits
Four groups of sources of the
water of hydrothermal solutions are distinguished
Hydrothermal deposits were formed over a
wide range of depths
Main types of hydrothermal ores
Vein Deposits
Hydrothermal deposits categorised
according depth and temperature
Gold Mineralisation
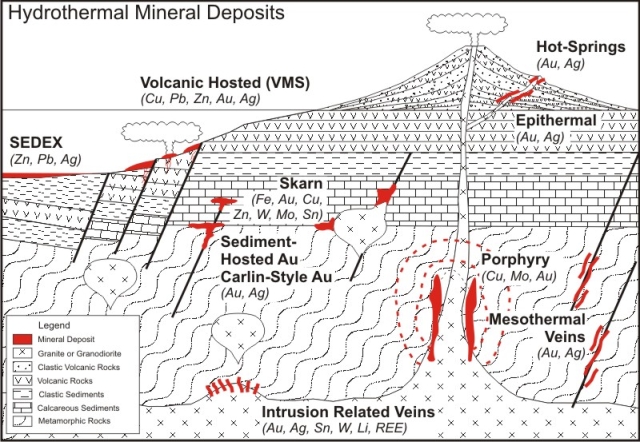
Hydrothermal Deposits a large group of mineral deposits formed from the sediments of hot aqueous solutions that circulate deep inside the earth.
Four groups of sources of the waterof hydrothermal solutions are distinguished:
(1) magmatic water, which separates from magmatic melts in the process of solidification and formation of igneous rock;
(2) metamorphic water, which is freed in the deep zones of the earth’s crust from water-containing minerals during their re-crystallization;
(3) water buried in the pores of marine sedimentary rock, which begins to move as a result of disturbances in the earth’s crust or under the influence of heat from within the earth; and
(4) meteoric water, which penetrates into the depths of the earth through water-permeable strata. The mineral substance found in the solution whose deposition forms hydrothermal deposits can be separated out by congealing magma or can be mobilized from the rocks through which subterranean waters are filtered.
Hydrothermal deposits were formed over a wide range of depths
—from the surface of the earth down to more than 10 km; the optimal conditions for their formation occur at a depth of several hundred meters to 5 km.The initial temperature of this process can be 700°-600° C, gradually decreasing to 50°-25° C; the most abundant formation of hydrothermal ore takes place in the range of 400°-100° C.
In the early stage, the water existed as steam, which condensed during gradual cooling and passed into the liquid state.
This was a true ionic solution of complex compounds of various elements, which precipitated out upon changes in pressure, temperature, and acid-alkali and oxidation-reduction characteristics.
The deposition of these elements could occur in open cavities and as a result of the replacement of rock through which hydro-thermal solutions flowed; in the first case mineral veins appeared, and in the second case, the mineral deposits took the form of metasomatic bodies. The most widespread form of hydrothermal bodies are veins, stockworks, and stratified and irregularly shaped deposits. They reach a length of several kilometers and a width of several centimeters to dozens of meters.
Hydrothermal deposits are flanked by a halo of diffusion of its component elements (primary diffusion halos), whereas the adjoining rocks are hydrothermally transformed. The most common processes of hydrothermal transformation are silicification and alkali transformation, in which the introduction of potassium leads to the development of muscovite, sericite, and clay minerals and the action of sodium leads to the formation of albite.
The following main types of hydrothermal ores are distinguished according to the predominant minerals:
(1) sulfide ores, which form deposits of copper, zinc, lead, molybdenum, bismuth, nickel, cobalt, antimony, and mercury;(2) oxide ores, which are typical for deposits of iron, tungsten, tantalum, niobium, tin, and uranium;
(3) carbonaceous ores, which are found in certain deposits of iron and manganese;
(4) native ores, which are characteristic of gold and silver; and
(5) silicate ores, which create deposits of nonmetallic minerals (asbestos and mica) and some deposits of rare metals (beryllium, lithium, thorium, and rare earths).
Hydrothermal ores are distinguished by their large number of component minerals.
They are usually unevenly distributed in the contours of ore bodies, forming alternating zones of high and low concentration that determine the primary mineral and geochemical zonality of the deposits.
There are several variants of genetic classifications.
In 1907 the American geologist W. Lindgren proposed a division into three classes, taking account of depth and temperature of formation (hypothermal, mesothermal, and epithermal).
In 1940 another American geologist, A. Bateman, noted two classes of deposits:
(1)those laid down in cavities and
(2) those formed by replacement.
In 1941, the Swiss geologist P. Niggli divided these deposits according to their characteristics in relation to magmatic rocks and their temperature of formation.
The Soviet geologist M. A. Usov (1931) and the German geologist H. Schneiderhöhn (1950) distributed hydrothermal deposits according to the level of solidification of the ore-bearing magmas.
The Soviet geologists S. S. Smirnov (1937) and Iu. A. Bilibin (1950) grouped hydrothermal deposits according to their connection with tectonomagmatic complex igneous rocks.
V. I. Smirnov (1965) proposed a grouping of hydrothermal deposits according to the natural associations of their component mineral complexes, which would reflect their genesis.
Hydrothermal deposits are of enormous significance in the extraction of many important minerals. They are essential for the production of nonferrous, rare, noble, and radioactive metals. In addition, hydrothermal deposits serve as the source of asbestos, magnesite, fluorspar, barite, crystal, Iceland spar, graphite, and several precious stones (tourmaline, topaz, and beryllium).
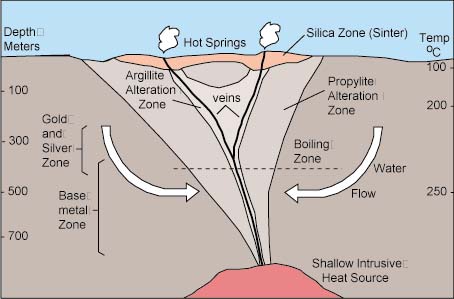
Vein Deposits
Veins are mineral deposits which form when a preexisting fracture or fissure within a host rock is filled with new mineral material. The deposition of minerals is typically performed by circulating aqueous solutions. Many ore deposits of economic importance occur in veins.Vein deposits are believed to form when aqueous solutions carrying various elements migrate through fissures in rock and deposit their burden onto the fissure walls. Hot, rising water escaping from cooling igneous plutons may deposit minerals as it ascends through the crust. As heated magmatic waters rise, the temperature and pressure of their environment drop and minerals exsolute and crystallize. Meteoric ground water may also percolate down through the earth's crust, dissolving surface minerals and gaining heat from the geothermal gradient or from nearby igneous intrusions. At greater depths the dissolved substances may precipitate and crystallize along the walls of the fissures and cavities through which the water travels.
Most vein deposits are formed as new mineral species are precipitated onto rock walls which themselves remain unaltered. In such cases mineral deposits fill the original crack or fissure in the host rock but do not extend into the host rock itself. The boundary betwen host rock wall and deposited vein minerals therefore remains clearly delineated. Vein deposits of this nature are a type of hydrothermal deposit because the mineral species which compose the veins were precipitated by hot waters.
However, sometimes the preexisting rock wall which contains the vein undergoes alteration. Portions of the host rock may either dissolve and be transported away or else react chemically with the circulating volatile fluids or the newly formed mineral species. In this case the boundary between vein deposit and original rock wall will be unclear. If most of the mineralization process occurs within the space once occupied by unaltered wall rock then the vein is termed ahydrothermal replacement deposit. A hydrothermal replacement deposit occurs when hot circulating aqueous solutions replace the original rock with new mineral species. This typically occurs in more soluble rocks such as limestone.
Hydrothermal replacement deposits are a form of hydrothermal metamorphism or metasomatism.
Hydrothermal deposits are categorised according to the depth and temperature at which they formed.
Some mineral species crystallize mainly at preferred temperatures and pressures. Because the temperatures and pressures are different for each type of hydrothermal deposit, each has a different, characteristic set of associated minerals.
Hypothermal
Hypothermal deposits are formed at great depths and high pressures and temperatures. Temperatures may range from 300° to 500° Celsius during the formation of such deposits. Casseterite, wolframite and molybdenum veins; gold-quartz veins; copper-tourmaline veins; and lead-tourmaline veins provide examples of mineral associations which may occur in hypothermal deposits. Minerals which are found in hypothermal veins include quartz, fluorite, tourmaline, and topaz. Ore minerals found may include native gold (Au); the sulfides galena (PbS), chalcopyrite (CuFeS2), pyrite (FeS2), molybdenite (MoS2), bismuthinite (Bi2S3), and arsenopyrite (FeAsS); the oxides uraninite (UO2), cassiterite (SnO2), and magnetite (Fe3O4); and the tungstates wolframite ((Fe,Mn)WO4) and scheelite (CaWO4). Metals which may be extracted from hypothermal deposits consist of copper (Cu), molybdenum (Mo), tin (Sn), tungsten (W), gold (Au), and lead (Pb).
Mesothermal
Mesothermal deposits form at intermediate depths, temperatures, and pressures. Temperatures may range from 200° to 300° Celsius during the formation of such deposits. Quartz and carbonate minerals such as calcite (CaCO3), ankerite (CaFe(CO3)2), siderite (FeCO3), dolomite (CaMg(CO3)2), and rhodocrosite (MnCO3) occur in mesothermal deposits. Ore minerals which may be found include native gold (Au) and the sulfides galena (PbS), sphalerite (ZnS), chalcopyrite (CuFeS2), pyrite (FeS2), bornite (Cu5FeS4), arsenopyrite (FeAsS), and tetrahedrite ((Cu,Ag)12Sb4S13). Metals which are mined consist of copper (Cu), zinc (Zn), silver (Ag), gold (Au), and lead (Pb).
Epithermal
Epithermal deposits form at shallow depths under relatively low temperatures and pressures. Temperatures during formation may range from 50° to 200° Celsius. Minerals found include quartz, opal, and chalcedony (SiO2); calcite (CaCO3), aragonite (CaCO3), and dolomite (CaMg(CO3)2); the halides fluorite (CaF2) and chlorargyrite (AgCl); the sulfate barite (BaSO4); native gold (Au); and the sulfides realgar (AsS), cinnabar (HgS), acanthite (Ag2S), pyrite (FeS2), orpiment (As2S3), stibnite (Sb2S3), proustite (Ag3AsS3), and pyrargyrite (Ag3SbS3). Metals which are mined from epithermal deposits include silver (Ag), gold (Au), and mercury (Hg).
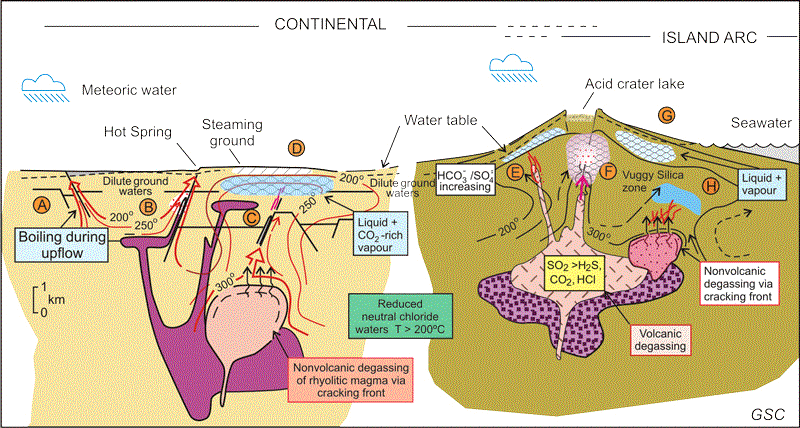
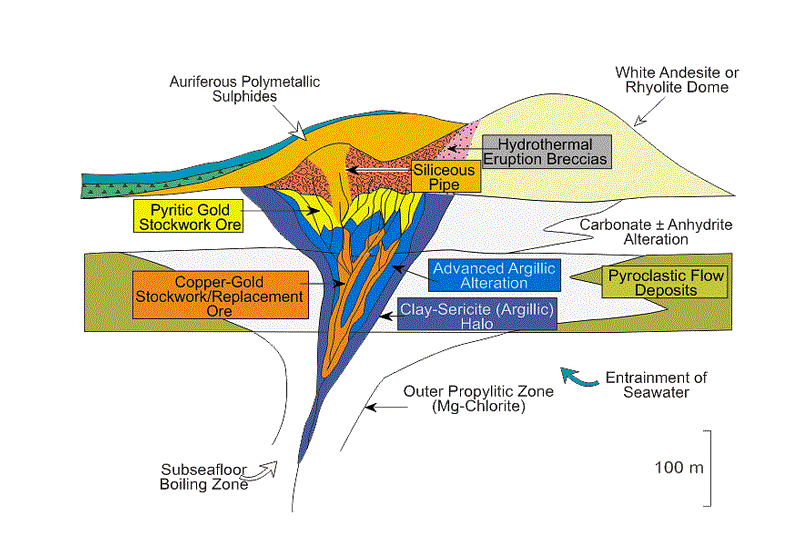
Gold Mineralisation
The association of gold mineralization with volcanic and geothermal hot spring activity has long been recognized by prospectors and geologists.We now know that this association is a consequence of the hot magmas which not only produce volcanic eruptions and volcanic rocks but also are the source of the hot fluids that transport gold and other metals and may in fact be the source of gold itself. Fluids emanating from a molten magma are extremely hot and under high pressure deep below the surface.
As these fluids rise, they mix with surface waters and change the composition of the rocks with which they come into contact. This process is known as alteration.
Eventually the fluids breach the surface and form either acidic lakes known as fumaroles common in the craters of volcanoes or dilute, neutral hot springs like those at Yellowstone or the Geysers in California. These two different surface manifestations – acidic lakes or neutral hot springs – reflect two different fluid types that each result from the two different paths taken by the magma as it rises to the surface. Both form gold deposits and are known respectively as low- and high-sulphidation gold deposits. In both subtypes gold will largely be precipitated from 2.5 kilometers depth to surface.
Recognizing that gold precipitates near the surface in these systems, the great American geologist Waldemar Lindgren coined the term epithermal in 1933, epi meaning shallow and thermal referring to the heated fluid. The chemist Werner Giggenbach further subdivided epithermal gold deposits into low and high sulphidation types (illustrated right1). Low and high do not refer to each type’s relative amount of sulphide minerals (metal complexes of sulfur with metals). Rather the distinction is based on the different sulfur to metal ratio within the sulphide minerals of each subtype. While this discussion deals with high-sulphidation epithermal systems, it is worth mentioning that low-sulphidation systems also form economic gold deposits although they develop under vastly different chemical conditions.

