Biofuels in Australia
Biofuels in Australia
sources CSIRO download here... BiofuelsinAustralia_CSIRO.pdfhttp://www.wvodesigns.com/wiki/Biodiesel
What is biodiesel?
Key Advantages of Biodiesel
Properties of Biodiesel
Biofuels - Alcohol an Overview
Power
Oil Producing Plants
Fuel security in Australia
Greenhouse gas emissions
Some
Longer Chain Hydrocarbons That May Have Potential As Bio-fuels

What is biodiesel?
Technically, biodiesel is Vegetable Oil Methyl Ester. It is formed by removing the triglyceride molecule from vegetable oil in the form of glycerin (soap). Once the glycerin is removed from the oil, the remaining molecules are, to a diesel engine, similar to petroleum diesel fuel. There are some notable differences. The biodiesel molecules are very simple hydrocarbon chains, containing no sulfur, ring molecules or aromatics associated with fossil fuels. Biodiesel is made up of almost 10% oxygen, making it a naturally "oxygenated" fuel.Biodiesel is the name for a variety of ester-based oxygenated fuels made from soybean oil or other vegetable oils or animal fats. The concept of using vegetable oil as a fuel dates back to 1895 when Dr. Rudolf Diesel developed the first diesel engine to run on vegetable oil. Diesel demonstrated his engine at the World Exhibition in Paris in 1900 using peanut oil as fuel.
Key Advantages of Biodiesel:
- is the only alternative fuel that runs in any conventional, unmodified diesel engine. It can be stored anywhere that petroleum diesel fuel is stored.
- can be used alone or mixed in any ratio with petroleum diesel fuel. The most common blend is a mix of 20% biodiesel with 80% petroleum diesel, or "B20."
- the lifecycle production and use of biodiesel produces approximately 80% less carbon dioxide emissions, and almost 100% less sulfur dioxide. Combustion of biodiesel alone provides over a 90% reduction in total unburned hydrocarbons, and a 75-90% reduction in aromatic hydrocarbons.
- provides significant reductions in particulates and carbon monoxide than petroleum diesel fuel.
- provides a slight increase or decrease in nitrogen oxides depending on engine family and testing procedures. Based on Ames Mutagenicity tests, biodiesel provides a 90% reduction in cancer risks.
- is 11% oxygen by weight and contains no sulfur. The use of biodiesel can extend the life of diesel engines because it is more lubricating than petroleum diesel fuel, while fuel consumption, auto ignition, power output, and engine torque are relatively unaffected by biodiesel.
- is safe to handle and transport because it is as biodegradable as sugar, 10 times less toxic than table salt, and has a high flashpoint of about 300 F compared to petroleum diesel fuel, which has a flash point of 125 F.
- can be made from domestically produced, renewable oilseed crops such as soybeans.
- is a proven fuel with decades of use
- When burned in a diesel engine, biodiesel replaces the exhaust odor of petroleum diesel with the pleasant smell of popcorn or french fries.
Properties of Biodiesel
Biodiesel is a better lubricant than modern low-sulfur petroleum diesel fuels, and it also has a much higher cetane rating. Cetane rating for diesel fuels is the equivalent of “octane rating” for petrol. The use of biodiesel generally reduces friction and wear on vehicle fuel systems. As well, biodiesel increases the lifespan of diesel engines with high-pressure injection pumps, fuel injectors or unit injectors which rely on fuel for lubrication in those parts.At room temperature, biodiesel is a free-flowing liquid. Depending upon the feedstock, its color varies between golden yellow to dark brown. It is very slightly miscible in water. It has a low vapor pressure and a high boiling point. Biodiesel’s flash point is at 130°C/266°. 28 This is significantly higher than the flash point of either petroleum diesel or petrol.
The density of biodiesel is around 0.88 g/cm³, which is slightly higher than that of petroleum diesel. Biodiesel generally has very little sulfur, so it is frequently added to Ultra-Low Sulfur Diesel fuels (ULSD) to provide lubrication, since sulfur compounds are responsible for most of the lubrication in petroleum diesel.
Fuel efficiency and caloric values
The caloric value of typical biodiesel averages about 37.25 MJ/kg. This is approximately 10% lower than regular No. 2 petroleum diesel. Although variations in biodiesel energy values are more dependent on the feedstock source than the production process, these variations are lower than those for petroleum diesel.Overall, biodiesel is said to offer better lubrication and more thorough combustion than petrodiesel, which may compensate for the biodiesel’s lower energy values.
The thermal efficiency and power output of biodiesel depends mostly on the load conditions when burnt, and the quality of the blend. The quality of the blend varies according to its specific density, viscosity and flash point. For example, thermal efficiency of B100 when compared with B20 varies according to the BTU values of the components of the blends.
Biodiesel standards are specified by the American Society for Testing and Materials (ASTM). This organization has performed extensive studies on the brake thermal efficiency of biodiesel blends under various load conditions using different compression ratios. Trials comparing B40 to petroleum diesel found that B40 provides greater efficiencies than petrodiesel at higher compression ratios.
It was found that as compression ratios are increased, the efficiencies of all fuel types and blends are increased. In summary, these studies found that a B40 blend was most economical with a compression ratio of about 21:1 when compared with other blends. This implies that increased efficiency of biodiesel follows from viscosity, fuel density, and heating value.
Combustibility & emissions characteristics
In most cases, fuels systems in modern diesel engines are designed to operate with petroleum diesel, not biodiesel. For example, pressures within direct-injection fuel systems may range from 3,000 psi for common applications up to as much as 30,000 psi for rail locomotive systems. In most cases, conversion from petroleum diesel to biodiesel requires modification of the fuel system.When compared with modern high-pressure fuel injection systems, traditional inline injection systems are more tolerant of poorer-quality diesel fuels. Yet, higher pressures and closer tolerances of high-pressure fuel systems provides better control over atomization and combustion, therefore offering greater efficiencies and fewer emissions.
In comparing emissions from petroleum diesel and biodiesel, one research study of fuel-injection systems examined the characteristics of droplets during the fuel-atomization process. It found that biodiesel produced larger-diameter droplets than those of petrodiesel. These larger droplets were attributed to biodiesel’s higher surface tension and viscosity. Also, it was found that B100 offers the greatest penetration of spray, which was attributed to its high density.
Large droplet size can cause inefficiency in combustion, increase emissions, and decrease power output. And, other studies have also noted a brief delay in the injection period of biodiesel. And, biodiesel’s greater viscosity and higher cetane rating also contribute to poor atomization as well as reduced air penetration during the pre-ignition period. However, another study indicated that biodiesel’s ignition-delay period may help reduce nitric oxide (NOX) emissions.
In addition to NOX, the combustion of diesel fuels produces other emissions which are also subject to regulation under the Environmental Protection Agency (EPA). In order to comply with regulations, biodiesel fuel systems must operate in such a way as to control the combustion process and mitigate its emissions.
Several recently-developed technologies are helping to control emissions from both petrodiesel and biodiesel engines. The exhaust gas recirculation system (EGR) and diesel particulate filter (DPF) are designed to reduce harmful emissions from diesel engines. The DPF uses potassium and sodium carbonates to aid in the catalytic conversion of combustion ash. And, studies have shown that when using EGRs, biodiesel has an advantage over petroleum diesel for reducing NOX emissions.
Compatibility with plastic, metal and rubber
Biodiesel is compatible when used with high-density polyethylene (HDPE) tubing, gaskets and valves, yet it slowly deteriorates parts made of polyvinyl chloride (PVC). And, polystyrene dissolves immediately on contact with biodiesel.With regard to metals, biodiesel has a corrosive effect on copper-containing metals such as brass. Biodiesel also has a slow adverse effect on tin, zinc, lead and cast iron. However, biodiesel does not affect stainless steels (Standards 304 and 316) and aluminum.
Biodiesel degrades parts made of natural rubbers, which are generally only found in older engines and furnaces. And, some studies have found that biodiesel which has lost its stability through oxidation may degrade fluorinated elastomers (FKM or FPM) which were cured with base-metal oxides and peroxides.
Still, the commonly-used synthetic rubbers used in today’s vehicles, such as FKM-GBL-S and FKM-GF-S, are unaffected by biodiesel under all operating conditions.
Gelling properties at low temperature
If biodiesel fuel is cooled to a certain temperature, some of its molecules clump together and become crystals. Biodiesel begins to appear cloudy when these crystals become bigger than one-quarter the size of visible light’s wavelengths. This is called the “cloud point” (CP). With further cooling of the biodiesel, the crystals grow larger.
As measured by the standard tests mentioned earlier, the “cold filter plugging point” (or CFPP) is the lowest possible temperature at which biodiesel fuel can still pass through a filter with 45-micrometer mesh size. If cooled further, biodiesel will gel and eventually solidify. As mentioned previously, CFPP requirements depend upon the climate region where the biodiesel fuel is to be sold.
The gelling temperature for pure biodiesel (B100) varies widely depending upon the exact mix of esters, which in turn depends on the feedstock from which the biodiesel was produced.
As an example, biodiesel fuel produced from canola seed containing low levels of erucic acid begins to gel at about 14°F (−10 °C). In contrast, biodiesel made from tallow or yellow grease containing animal fat begins to gel at about 61°F (+16 °C).
There are several commercially-available additives which serve to counteract gelling by lowering the CFPP and the temperature at which biodiesel can be poured. Even during winter in northern climates, biodiesel is still viable as a fuel when blended with other fuel oils such as Number 2 low-sulfur diesel and Number 1 diesel-kerosene.
Another method for using biodiesel fuel under cold conditions is by installing a second fuel tank for biodiesel to be used in conjunction with a standard petroleum diesel fuel source. This second tank is insulated, and the biodiesel inside is warmed with a heating coil fed by engine coolant.
Once the already-operating engine has warmed the biodiesel enough to flow properly, the fuel source can be switched over from petrodiesel to biodiesel. Likewise, pre-heating can also be used to fuel diesel engines by using virgin vegetable oil or waste vegetable oil (WVO) instead of diesel.
Contamination with water and other substances
Biodiesel often contains small yet problematic amounts of water. Even though biodiesel is barely miscible with water, still, biodiesel is hygroscopic, which means that it actively attracts water.Biodiesel absorbs water because it contains mono- and diglycerides which remain after the production process. These glycerides act as an emulsifier and hold water in the biodiesel. As well, biodiesel may also contain residual water from condensation in storage tanks.
Water is problematic in biodiesel for several reasons: It creates smoke in the exhaust, and it reduces the heat and therefore the power of combustion. It also corrodes fuel-delivery components and causes pitting within the engine’s combustion chambers.
Water can also deteriorate paper-filter elements. And, in cold climates water freezes into ice crystals which accelerate gelling of the biodiesel fuel.
The presence of water has also been a problem during biodiesel production when using chemical catalysts, since it reduces the efficiency of high-pH agents such as potassium hydroxide. However, some biodiesel production methods, such as the super-critical methanol method, are unaffected by water contamination.
In the past, it was difficult to precisely measure the amount of water in biodiesel using field tests, but that task has now been made easier by the use of water-in-oil sensors.
Centrifuging to remove impurities from biodiesel and waste oils
Contamination from water and particulate matter in biodiesel and waste oils such as waste vegetable oil (WVO) and waste motor oil (WMO) is often removed with centrifuges. Many small producers of biofuels rely on centrifuge units which quickly clean waste vegetable oil, waste motor oil and contaminated biodiesel. These centrifuges efficiently remove water and other contaminants.Availability and pricing of biodiesel
In the United States, in May 2013, monthly biodiesel production reached a record high level of 111 million gallons.In certain countries under government-subsidized conditions, biodiesel costs may be less than petroleum diesel. In the U.S., according to recent U.S. Government pricing reports for January 2014, the average pump price for B20 biodiesel was $3.97 per gallon, and B99-B100 biodiesel was $4.28 per gallon.
In comparison, during the same reporting period the average price for petroleum diesel was $3.89 per gallon, and standard petrol was $3.34 per gallon.
Biodiesel feedstocks & production methods
Many different feedstocks can be used to make biodiesel. Common feedstocks are: Virgin plant oil; waste oil, including waste vegetable oil (WVO) and used cooking oil; animal fats, such as tallow and lard; yellow grease, restaurant grease and other mixtures that include animal fats; algae and halophytes; and sewage.Biodiesel is produced through transesterification processes. Over time, various methods have been developed for the transesterification reaction, such as the common batch process, various supercritical methods, ultrasonic processes, and microwave processes.
Transesterified biodiesel is mostly composed of a mixture of mono-alkyl esters from long-chain fatty acides. The most common reaction process uses methanol (in the form of sodium methoxide) as the cheapest alcohol source in order to provide methyl esters. This method is called the Fatty Acid Methyl Ester (FAME) process.
The methanol used during biodiesel reaction processes is most often produced using fossil fuels. Yet, there are also some “greener” sources of methanol made from biomass, glycerol or carbon dioxide as feedstock.
Likewise, ethanol can be used to produce biodiesel through ethyl esters. This is called the Fatty Acid Ethyl Ester (FAEE) process. By using alcohols with higher molecular weights, the resulting biodiesel product has better cold-flow properties, yet the transesterification reaction is less efficient.
Through whichever method, the base oil is converted into the desired esters by means of a lipid transesterification process. Remaining free fatty acids (also known as FFAs) in the oil are converted into soap and taken out during the process, or else they are later esterified using an acid catalyst, which yields further biodiesel product.
By undergoing esterification, biodiesel becomes much different from ordinary vegetable oil. Once this process is complete, biodiesel possesses combustion properties very similar to petrodiesel and can be used to replace it in most applications.
Glycerol is a byproduct of the transesterification process. As an approximate ratio, along with each ton of biodiesel manufactured about one hundred kilograms of glycerol are also produced.
During the early years of biodiesel production, glycerol was considered a valuable byproduct. Yet, because of the increasing global production of biodiesel the price of crude glycerol has fallen considerably.
Still, there are uses for glycerol, both as a feedstock for glycerin (used primarily in the cosmetic and pharmaceutical industries) as well as being a building block for other oleochemical products.
Although the purification of crude glycerol is an energy-intensive process, beyond glycerin it can also be used to make valuable products such as propylene glycol , as well as epichlorhydrin, which is a source for epoxy resins. In any event, the use of these byproducts from biodiesel processes provides new sources for chemicals from non-petroleum feedstocks.
Rapeseed and soybean oil feedstocks
In the U.S., virgin plant oils are the most-common feedstocks for biodiesel production. Rapeseed and soybean oils are the leading sources, with soybean oil representing about half of all U.S. biodiesel production.Other virgin plant oil sources
Other virgin plant oil feedstocks include sunflower, palm, coconut, hemp, pennycress, jatropha, mustard, jojoba and pongamia, Corn, Cashew, Oat, Palm , Lupine, Rubber seed, Kenaf, Calendula, Cotton Hemp, Soybean, Olive tree, Castor bean, Pecan, Oil Palm, Coffee, Linseed, Hazelnut, Euphorbia, Pumpkin seed, Sesame Safflower, Rice, Sunflower, Peanut, Tung oil tree, , Macadamia nut Brazil nut, Avocado, Coconut, Macuba palm among others. Some nut trees in Papua New Guinea show particular promise.The primary factors used to determine whether a given plant oil is suitable as biodiesel feedstock include its flash point, viscosity and flow characteristics at low temperatures, energy content, and its combustion products and emissions.
As well, the cost of the plant oil is an important factor. Cost is based on yield as well as the resources required to grow the oil-bearing plant, then harvest and process it.
Another factor affecting the cost of biodiesel is whether the feedstock oil plant may be used for both food and biodiesel production. There is a complex marketplace effect on the prices of oil-bearing plants which may be used for both food and biodiesel production.
In contrast, some plant oils, such as jatropha and tung oil, are non-edible or toxic and therefore have only industrial uses such as for biofuel.
Biodiesel production from waste vegetable oil (WVO)
Waste vegetable oil (WVO) may be collected, filtered, centrifuged and burned directly as a fuel source for combustion engines and heating furnaces which have been converted for biodiesel use. Or, waste vegetable oil can be processed into biodiesel fuel. 51Nevertheless, with the availability of high-quality centrifuges many individual alternative-fuel users choose to simply collect and clean batches of waste vegetable oil, then use it directly to fuel their diesel vehicles converted to operate on WVO, instead of first producing biodiesel from that waste oil.
For individuals who wish to produce biodiesel instead of simply burning WVO, the following is a home-method for collecting, filtering and reacting waste vegetable oil into biodiesel. Before beginning this process, be sure to confirm that home-production of biodiesel is legal in a particular community.
Also, it’s important to take appropriate safety precautions at all times during this process. This includes keeping all materials and supplies beyond the reach of children and pets. As well, home-biodiesel producers should work with appropriate safety equipment in a well-ventilated area.
Chemical-resistant goggles, gloves and aprons are necessary. An appropriate work area will include a source for running water, an eye-wash station, at least one fire extinguisher, supplies for cleaning up spills, and a telephone in case of emergency.
The first step in producing biodiesel at home is to collect waste vegetable oil (WVO), usually in the form of used cooking oil gathered from restaurants and industrial food-processing sources which rely on fryers. The collected WVO should be filtered to remove large particles.
Chunks or particles of fryer residue may contain water, which causes problems during the biodiesel reaction process. Also, the liquid should be allowed to settle after filtering, so smaller particles will move to the bottom of the container. Particles may interfere with the heating phase during the biodiesel process. After settling, the filtered waste vegetable oil is transferred into the reaction tank.
In the reaction tank, the WVO must be heated to about 120 or 130°. Also, the waste vegetable oil must be circulated during the heating process, in order to reduce the chance of overheating near the heating element.
Heating and circulation may take between one to four hours, depending on the quantity of oil being treated, the air temperature, the overall oil temperature, the amount of insulation around the tank, and the power of the heating element.
For each liter of waste vegetable oil, the biodiesel reaction process requires the use of 200 ml. of methanol and 3.5 grams of lye, as well as suitable glass or non-reactive containers and blending apparatus.
The first step is to place the methanol into a glass container. Next, activate the blender so that the methanol is being stirred. Next, add the 3.5 grams of lye for each 200 milliliters of methanol. This process should be conducted in a dry environment, since the lye can absorb water from the air. Keep the lye container sealed tightly between uses.
The reaction between lye and methanol creates sodium methoxide. This should be used for reaction with the waste vegetable oil fairly quickly, since sodium methoxide degrades in the presence of moisture and air.
With mixing, the methanol should completely dissolve the lye within a few minutes. Once the lye is dissolved, proceed quickly to the following step. Concurrently with the above procedure, the waste vegetable oil should be heated in a separate container to 130°F (or 55°C). Carefully add the hot WVO to the lye-dissolved-in-methanol mixture. Mix for between 20 to 30 minutes to complete the reaction process.
During the reaction process, biodiesel and glycerin are formed, and separate into two distinct layers in the container. The mix will result in about 90% biodiesel and 10% glycerin. As the mix cools and settles, it will separate into two layers. Since the biodiesel has lower density than the glycerin, the biodiesel will float on top.
The biodiesel top layer can be carefully removed using a pump or baster, leaving the lower glycerin behind. The biodiesel should be stored appropriately, and the glycerin can either be poured on a compost heap to enrich the decomposition, or it can be used to make soap.
Given the availability of centrifuges, many individual users choose to simply convert and operate their vehicles using waste vegetable oil (WVO) instead of biodiesel.
Biodiesel production from animal fats: Tallow, lard, yellow grease, chicken fat, fish oil, and other animal feedstocks
Biodiesel fuel can be produced from animals fats using a process similar to that described above for waste vegetable oil. Although biodiesel based on animal fat is sometimes perceived as being of lower quality than biodiesel from waste vegetable oil, this is not necessarily true.Biodiesel produced from either plant oils or animal fats contains the same combustible chemicals, yet the proportions vary in each type. Both types are biodegradable, renewable energy sources, so they are better choices than petroleum-based diesel fuel.
All biodiesel fuels may be sluggish in cold-weather conditions regardless of feedstock. And, animal fat-based biodiesel has a higher cloud point and freezing temperature than plant oil-based biodiesel. Still, extensive tests have shown that B5 blends containing animal fat-based biodiesel do not negatively affect cold-weather performance.
Although animal fat-based biodiesel had a bad reputation in the early years of the biodiesel industry due to poor refinery practices, nowadays biodiesel from animal fats is of excellent quality if it complies with the applicable ASTM and/or EN specifications.
For example, biodiesel made from animal fats typically offers a higher cetane number. As indicated in an earlier section of this article, biodiesel’s cetane number indicates the ignition quality of a diesel fuel.
Biodiesel produced from soy oil averages between 45 and 53, and petroleum diesel averages between 41 and 53. In comparison, the cetane rating of animal fat-based biodiesel averages between 55 and 61. Beyond improving combustion, a higher cetane number usually indicates reduced emissions of NOx and particulates. As well, biodiesel made from animal fats also offers better lubricity and protection for moving engine parts.
Animal fat-based biodiesel offers an advantage in oxidative stability when compared with plant oil-based biodiesel. Biodiesel in general may form sediments while being stored. Yet, the high percentage of saturated fats in animal-sourced biodiesel offers better oxidative stability, which reduces the amount of sedimentation.
As further evidence of the comparative advantages of biodiesel produced from animal fats instead of plant oils, some studies have shown that animal fat-based biodiesel reduces greenhouse gas emissions because it is based entirely on byproducts, whereas much biodiesel is produced from virgin plant oils.
And, while meat production in the U.S. continues to grow at a slow yet steady pace, currently less than 10% of the available waste animal fat and tallow are used for making biodiesel. So, biodiesel production based on animal fats offers continuing opportunity for growth.
Algae and halophytes
Algae (singular ‘alga’) are a large, diverse group of small plant-like organisms that live in aquatic environments. Algae include many forms, from single cells to giant, multicellular kelps. The usual definition of algae includes the fact that most forms rely on chlorophyll for photosynthesis as their source of energy.Algae are of great interest as a feedstock for biodiesel and other biofuels because they grow rapidly and they lack the typical hard, woody structure that characterizes most land-based plants. So, algae can be grown, harvested and processed with less-intensive inputs than land-based oil-bearing plants.
Algae can be grown in both freshwater and marine environments, so they do not require land as a resource. Algae can also be grown using sewage. As well, coastal marine plants such as halophytes (“salt-loving” plants) can be grown in salty areas without requiring land.
Studies have shown that the per-acre yields of algae and halophytes can equal or exceed the yields from land-based biodiesel sources such as soybeans and other oil-bearing plants.
Sewage sludge
Sewage sludge also shows commercial promise as a source for biodiesel.Quantity of feedstocks, yields and production levels
Many advocates have suggested that waste vegetable oil (WVO) is the best overall source of feedstock for biodiesel production.Still, the available supply of waste vegetable oil is significantly less than the quantity of petroleum-based biodiesel currently being used for combustion engines and home heating worldwide. So, whether converted into biodiesel or burned as-is, WVO is only a partial solution for global energy needs.
As mentioned earlier, animal fats are a growing source of feedstock for biodiesel production. Animal fats are byproducts of meat processing and cooking. Although it would be uneconomical to raise animals (or gather fish) solely for their fat content, still, the byproducts add significant value to livestock and fishing industries.
An increasing number of multi-feedstock facilities in the U.S. are producing biodiesel based on mixtures containing animal fats. Some facilities rely solely on chicken fat from large poultry producers.
As well, some biodiesel producers use byproduct fish oil as feedstock. In fact, fish can be a rich feedstock for biodiesel, since at least one facility has reported biodiesel yields of 13 tons from 81 tons of fish waste byproduct, beyond the primary yield of 130 tons of marketable processed fish.
Current research into the newest sources and methods for biodiesel
Because of concerns about deforestation, and the debate about whether plants which bear edible crops should be devoted to producing food for human consumption or fuel such biodiesel, some researchers have proposed that biofuels should be produced only from non-food sources such as algae or non-edible plants.Algal biodiesel
Algae grown in the marine coastal areas of the sea could provide biodiesel that would not displace land-based food crops. There are currently many research projects focused on developing cost-effective ways to grow algae for biofuel use. 61 In fact, some species of algae yield exceptionally high levels of oil, sometimes as great as 50% of algal volume.Pongamia

Pongamia, also known by its scientific name Millettia pinnata, is an oilseed-bearing tree which grows rapidly even under fairly harsh conditions. Pongamia is non-edible, and can be grown in non-arable conditions, so it is not considered a competitor for land on which food crops are grown. The tree appears to be an excellent candidate for biodiesel production because it has an exceptionally high oil yield, approximately 40% of the seed weight.
Jatropha and other poisonous plants
Jatropha is a poisonous shrub which produces oil-bearing seeds considered an excellent feedstock for biodiesel production. The plant grows on marginal land, so it is not expected to compete with food crops. Certain proprietary varieties of jatropha produce exceptional yields, and much preliminary development is now underway.Biodiesel in the future
Beyond using plant crops and algae as biodiesel feedstocks, there are a range of visionary research projects underway worldwide to discover better biofuel sources for the future. For example, in Ghana, Africa researchers are developing the world’s first biodiesel production plant to be fed by human fecal sludge. 65 And, the Spanish company Ecofasa is working to develop biodiesel made from household and garbage.As well, even though such feedstocks are not expected to solve the world’s energy needs, encouraging early research has shown that waste materials including used coffee grounds can be used to produce biodiesel.
Likewise, other exotic feedstocks can be used to produce good biodiesel. Studies have shown that biodiesel can be produced from alligator fat. Alligator fat is left as a byproduct of meat and skin industries in the U.S., and is currently being disposed of in landfills at the rate of about 15 million pounds per year.
These early efforts hold much future promise for reducing the world’s dependence on petroleum-based diesel and other fossil fuels, and may help consumers move toward a future in which the energy needs for combustion engines are fulfilled largely by renewable feedstocks such as waste vegetable oil (WVO) and biodiesel.
The big challenge for biodiesel is whether large enough quantities can be produced without impacting on world food supplies or degrading the planet's biodiversity by covering the the surface in endless expanses of monoculture.
Efficiency, economic arguments and economic impact of biodiesel
Soybeans and rapeseed are the United States’ top two biodiesel feedstocks. Beyond these and other virgin-oil biodiesel feedstocks, waste vegetable oil (WVO) and animal fats also contribute significant amounts of value to economies in the U.S. and elsewhere worldwide.Power
One of the major advantages is the fact that it can be used in existing engines and fuel injection equipment (no modification required) without negative impacts to operating performance.Fuel availability / economy
Virtually the same L/100Km rating as petrodiesel and the only alternative fuel for heavyweight vehicles requiring no special dispensing and storage equipment.
Storage
Readily blends and stays blended with petrodiesel so it can be stored and dispensed wherever diesel is stored or sold.
Combustibility/Safety
Biodiesel has a very high flash point (300°F) making it one of the safest of all alternative fuels.
Production/Refining
The only alternative fuel that can boast of a zero total emissions production facility
Lubricity
The only alternative fuel that can actually extend engine life because of its superior lubricating properties.
Environmental Impact
The only renewable alternative diesel fuel that actually reduces a major greenhouse gas components in the atmosphere .
The use of biodiesel will also reduce the following emissions:
- carbon monoxide
- ozone-forming-hydrocarbons
- hazardous diesel particulate
- acid rain-causing sulfur dioxide
- lifecycle carbon dioxide
Biofuel - Alcohol an overview
The first four alcohols, (in order of carbon content) methanol, ethanol,propanol, and butanol, are of greatest interest for fuel use as their chemical properties make them useful in internal combustion engines.
One of the problems with alcohols is that they have lower energy densities than petrol.
Fuel Energy Density(megajoules/liter)
Average Octane (AKI rating/RON) petrol ~33 85-96/90-105
Methanol ~16 98.65/108.7
Ethanol ~20 99.5/108.6
Propanol ~24 108/118
Butanol ~30
AKI - Anti-Knock Index:
This octane rating is used in countries like Canada and the United States. RON - Research Octane Number: This octane rating is used in Australia and most of Europe
Besides reducing knock, higher octane values are indicative of a fuel that burns slowly. In general, the slower a fuel burns, the more efficient it is to extract energy from it. Thus, a higher octane also reveals a more energy efficient fuel. The fact that alcohols have higher octane values than petrol helps to offset some of the difference in energy density. The net result is that the loss of fuel economy (how far a car can travel on a volume of fuel) is not as drastic as it would be if the octane numbers were the same.
It is true that any of the alcohols above can be generated from fossil fuels. However, it is easier and more efficient to derive these products from biomass or even carbon dioxide and water than to refine petroleum. It is also the case that petroleum-derived alcohols tend to be less pure (ethanol is contaminated with methanol for instance) and often cannot be purified through simple distillation.
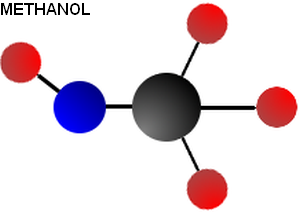
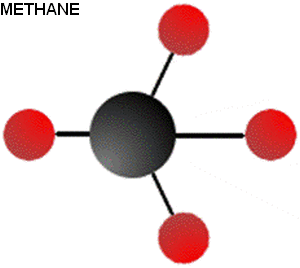
Methanol is closely related to methane. In fact, there is only one atom different between these two chemicals (Oxygen shown in blue, carbon in black, hydrogen in red)
Though these molecules are remarkably similar, their properties could not be more different. To begin, methane is a gas at standard temperature and pressure. Methanol, on the other hand, is a liquid. Methane has an energy density of 55 MJ/kg while methanol has an energy density of only 23 MJ/kg at best. Burning methane produces twice the amount of carbon dioxide per kilogram as burning methanol does (2.74 kg CO2/kg methane versus 1.37 for methanol).
Ethanol is standard drinking alcohol, so we know it isn't poisonous. Already we're off to a better start than methanol. It also has a higher energy density than methanol and is still a liquid, which makes it an attractive alternative. Ethanol is only one atom different than ethane. Like methane and methanol, the differences between these molecules are drastic.

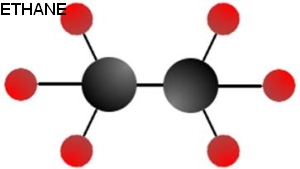
Ethanol is a common additive to fuels in many countries. Globally, the average ethanol content in petrol is roughly 5.4%, though some countries supply 25% or even 100% ethanol for vehicle fuel.
Ethanol has some benefits as a fuel or as a fuel additive. First, because it has a higher octane number than ethane and even many of the larger hydrocarbons, ethanol can be used to boost the octane of a fuel. Beyond that, it burns cleaner than most hydrocarbon fuels and, if created from biomass rather than petroleum, contains little or no contamination to damage vehicle parts
The drawbacks to ethanol as a fuel, however, have prevented its widespread adoption. First of all, it takes about 1.5 times more ethanol than petrol to get the same energy. That means you need a fuel tank 1.5 times larger if you want to travel the same distance on ethanol that you do on petrol. Another problem with ethanol is that it is corrosive to the rubber. Finally, ethanol absorbs water from the environment, which dilutes its concentration and makes it impossible to ship it through pipelines.
So, ethanol isn't the optimal standalone fuel, but it does seem to make for a great fuel additive
Propanol is the forgotten alcohol fuel, but for good reason. Propanol is the most difficult and expensive alcohol to produce.
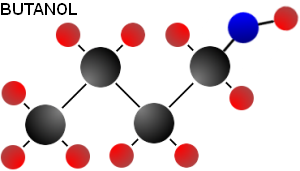
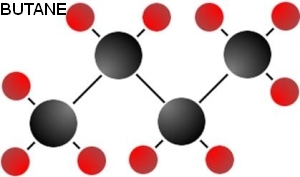
Butanol is more similar to petrol than ethanol or methanol. This similarity is a consequence of its longer hydrocarbon chain, which means there is more carbon in relation to the single oxygen and thus the molecule is less polar.
The similarity of butanol to petrol means that it can be used in a standard vehicle without the need for modifications. Also, because of its size, butanol has an energy density similar to that of petrol. In fact, butanol is so close to petrol in energy content that its octane rating nearly makes up for the difference in energy density. In other words, a liter of butanol will get your car about as far as a liter of petrol, with the difference only being about 10%. It is also true that butanol only produces about 2.03 kg of carbon dioxide per kilogram of butanol while petrol produces 3.3 kg of carbon dioxide per kilogram of petrol.
Three Reasons Why Butanol Has Not Replaced Petrol:
- Butanol actually produces more carbon dioxide than what arises just from burning it. The reason for this discrepancy is that producing the biomass, harvesting it, and processing it all requires energy which releases CO2
- Butanol's health effects are not well understood
- Butanol is very difficult to produce.
A better way to compare carbon dioxide production is in terms of energy produced per kilogram of carbon dioxide expelled. This is a better example because even though a quantity of ethanol produces less carbon dioxide than the same quantity of gasoline, it also produces less energy. The result is that it is necessary to burn more of the alcohol to get the same amount of energy as the fossil fuel. The chart below contains a few examples that help to clarify the point.
This measure compares an alcohol to its closest hydrocarbon equivalent.
The value of carbon produced is arrived at by determining how many kilograms of alcohol are needed to derive the same amount of energy as the hydrocarbon.
This number is multiplied by the CO2 production in kg/kg to determine how many kilograms carbon dioxide are produced for when energy production is kept the same.
Fuel |
Energy Density (MJ/kg) |
Carbon Dioxide production (kg/kg) |
Carbon Dioxide production (MJ/kg) |
Carbon Dioxide* (kg/Equivalent) |
|---|---|---|---|---|
Methanol |
~21 | 1.37 | ~15 | ~3.6 |
Methane |
~55 | 2.74 | ~20 | N/A |
Ethanol |
~24.5 | 1.91 | ~13 | ~4.05 |
Ethane |
52 | 2.93 | ~18 | N/A |
Butanol |
36 | 2.37 | ~15 | ~3.02 |
Petrol |
~46 | 3.30 | ~14 | N/A |
Longer Chain Alcohols Would Make Alcohol a More Viable Alternative to Fossil Fuels
So it would appear that alcohols, no matter how they are produced, are not likely to be viable alternatives to fossil fuels until they start to get longer chains.
Methanol and ethanol are not great fuel sources because they produce more carbon dioxide than their equivalent hydrocarbons for the same amount of energy.
Butanol, however, begins to show a difference.
Butanol produces LESS carbon dioxide than petrol for the same amount of energy but is currently difficult to produce in large quantities.
If we can produce butanol in large quantity and avoid impact on the food chain, then it may become a viable alternative to hydrocarbon fuels.
A method of genetic engineering has revealed that bacteria may be the key to producing long-chain alcohols.
Some Longer Chain Hydrocarbons That May Have Potential As Bio-fuels
Furfural C5H4O2
a distilled byproduct of plant lignocellulosic biomass including agricultural waste from corncobs, oat, wheat bran, and sawdust.
About 3% to 10% of the mass of crop residue feedstocks can be recovered as furfural, depending on the type of feedstock. Furfural and water evaporate together from the reaction mixture, and separate upon condensation.
2-Methyltetrahydrofuran
is an organic compound with the molecular formula C5H10O that is derived from furfural. Mixed with ehtanol it has potential as an alternative for fuel based vehicles. It has unusual properties , heating it reduces its ability to absorb water. Problems as a fuel appear to be it's density and toxicity.

