REE Rare Earth Elements in Australia
REE Rare Earth Elements in Australia :
REEs the 15 Rare Earth Elements +Scandium +Yttrium +Lutetium
Why Are REE's Different?
Listing of Rare Earth Elements Australia
Challenges to REE mining and extraction
Four (often overlapping) geological
environments:
Some Australian Possibilities
Processing of REE's
REE Economic Considerations
Sources
-----------------------------------------------------
Why Are REE's Different?
All of the REEs, except promethium, are more abundant on average in the Earth’s crust than silver, gold, or platinum. However, concentrated and economically minable deposits of REEs are unusual.
Found as REO (rare earth oxides) end-use markets (catalysts, glass industry, metallurgy excluding battery alloy, and phosphors) consume mainly cerium (45 percent), lanthanum (39 percent), and yttrium (8.0percent) oxides.
Dysprosium, gadolinium, neodymium, and praseodymium oxides and other REO's contribute the remaining 7.0 percent of total REOs consumed in these sectors.
Generally, atom radii get larger as atomic numbers increase due to the attachment of electrons to the outer shell, however, REEs display decreasing atom radii with the increase of atomic numbers. This phenomenon is known as lanthanide contraction.
So REEs have in general a greater amount of reactive area making them ideal for ...catalysts /anodes ; light-weight super-strong magnets, as colour phosphors for LEDs / display screens, as abrasives and energy converters.
REE resources in Australia
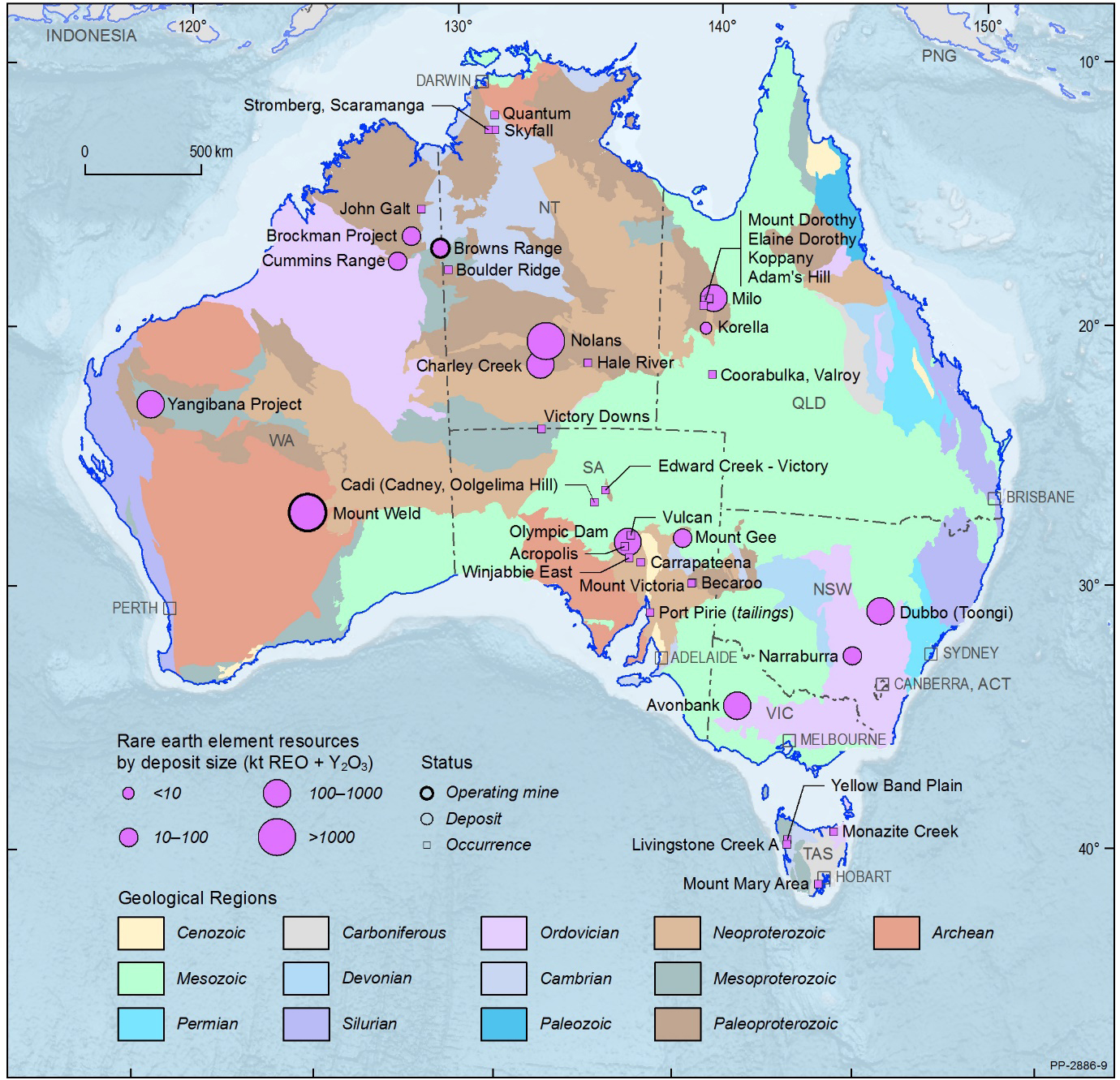
Listing of RE Rare Earth Elements in Australia
- Cerium (Ce) - 25th most abundant crustal element, more common than copper or lead, show a strong affinity for elements like phosphorus, making them suitable as water purifiers, precision glass polishing, devices requiring a carbon-arc, radiation resistant glass that absorbs ultraviolet radiation and release electrons into the glass matrix called photosensitive glass, red pigment, essential component of white LEDs emits green to yellow-green light
- Dyprosium (Dy) - can handle a greater saturation
magnetization than more common elements like iron, which allows for
fabrication of a stronger and smaller magnet particularly in
high-temperature magnets, used as protective coating in automotive
catalytic converters, high performance in electric motors such as in
surgical robots or wind turbines
- Erbium (Er) - used in the making of fiber optic cables and laser repeaters, used to colour the lenses of glasses, used in laser surgery
- Europium (Eu) - anti-counterfeiting phosphors for currency
security, can be used for both blue and red colours in LEDs and
displays
Gadolinium (Gd) - injecting a gadolinium-based contrast agent
into the patient’s bloodstream before MRI imaging enhances the
visibility of blood vessels, organs, and tissues , PET scanning uses
anti-body detecting molecule withn added gladolinium to locate for
example cancer cell concentrations. - Holmium (Ho) - very-high-power magnets used in microwaves and
nuclear control rods, converts magnetic energy into mechanical energy
- applications in sensors, actuators, and transducers, colours glass
red, doping agent in solid state lasers.
- Lanthanum (La) – digital cameras/phone lenses and infared
absorbent glass, because it has a great amount of reactive area within
its own mineral structure used as additives to refine hydrocarbons,
acts as a hydrogen absorber in rechargeable batteries, several
kilograms used for the anode of Nickel-metal hydride battery in hybrid
cars, used for storage in hydrogen-power cars, lanthanum-barium
radiometric dating
- Lutetium (Lu) - the scarcest REE, the 60th most abundant
crustal element, cracking hydrocarbons
- Neodymium (Nd) – Nd–Fe-B magnets are the strongest magnet
known, high performance in electric motors such as in surgical robots
or wind turbines, magnets are three times stronger, and one-tenth the
size of conventional magnets, used in tiny smartphone microphones,
infared lasers
- Praseodymium (Pr) - high-power praseodyniummagnets with Nd,
Every Electric Vehicle drivetrain requires up to 2kg of
neodymium-praseodymium oxide — but a three-megawatt direct drive wind
turbine uses 600kg.
- Promethium (Pm) - is the only rare earth element, which does not occur naturally,used in lasers which are used to communicate with submerged submarines, atomic batteries, Promethium-147 is used in very small quantity in Philips CFL glow switches
- Samarium (Sm) - high-temperature permanent magnets, warm white-light LEDs
- Scandium (Sc) – aerospace aluminium alloys for turbine
blades, solid oxide fuel cells converts chemical energy into
electrical energy with high efficiency, specialised lighting,
ceramics, lasers, electromics, as a catalyst in fuel cells
- Terbium (Tb) - high-temperature magnets, blue phosphor used
LED, used in sonar systems
- Thulium (Tm) - when bombarded in a nuclear reactor can be
used as a radiation source in portable X-ray instruments, used to
detect faults in inaccessible electronic and mechanical components
- Ytterbium (Yb) - used in the world’s most stable atomic
clock, alloyed to improve stainless steel, used in the making of
memory devices and tunable lasers.used in the making of memory devices
and tunable lasers, amplifies signal in optic fibres
- yttrium (Y) – ceramics, wear-resistant cutting tools, high
temperature, red phosphor used in LED, superconductors, abrasives,
bearings, seals
- Cerium, lanthanum, neodymium, and praseodymium, commonly in
the form of a mixed oxide known as mischmetal, are used in
steel making to remove impurities and in the production of special
alloys

Note: REO (rare earth oxides) are reported by companies as ...
TREO (total rare earth oxides) + yttrium oxide (Y2O3)
Challenges to REE mining and extraction...
- often very low concentrations
- often very small, spotty and irregular deposits
- found together with other REE’s - complex to separate
- often found with apatite, vermiculite, Cu, Ti, fluorite, Th, U, natural zirconia, and Fe
- presence of radioactive Uranium and Thorium complicate concentration / waste processes
- association with carbonatites or uranium / copper concentrations
- tailings from former / existing uranium / copper / fluorite / zircon resources may be reworked
Four (often overlapping) geological environments:
--> carbonatites
--> alkaline magmas
--> placer deposits
--> ion-adsorption clay deposits
1. Carbonatites are mostly emplaced in continental extensional settings and range in age from Archean to recent.
Coexisting with alkaline silicate igneous rocks they form alkaline-carbonatite complexes, but also occur as isolated pipes, sills, dikes, plugs, lava flows, and pyroclastic blankets.
These cone sheets, ring dikes, radial dikes, and fenitisation-type halos (advancing alteration by CO2 carbon dioxide) carbonatites are the premier source for light rare earth element (LREE) deposits.
LREEs consist of La, Ce, Pr, and Nd of which Nd and Pr are particularly marketable.
In carbonatite melts, which are either directly mantle-derived or immiscible from silicate melts, alkalis and LREEs concentrate in the residual melt due to their incompatibility in early crystalising minerals. In most carbonatites, additional fractionation of calcite or ferroan dolomite leads to evolution of the residual liquid into a mobile alkaline “brine-melt” from which primary alkali REE carbonates can form.
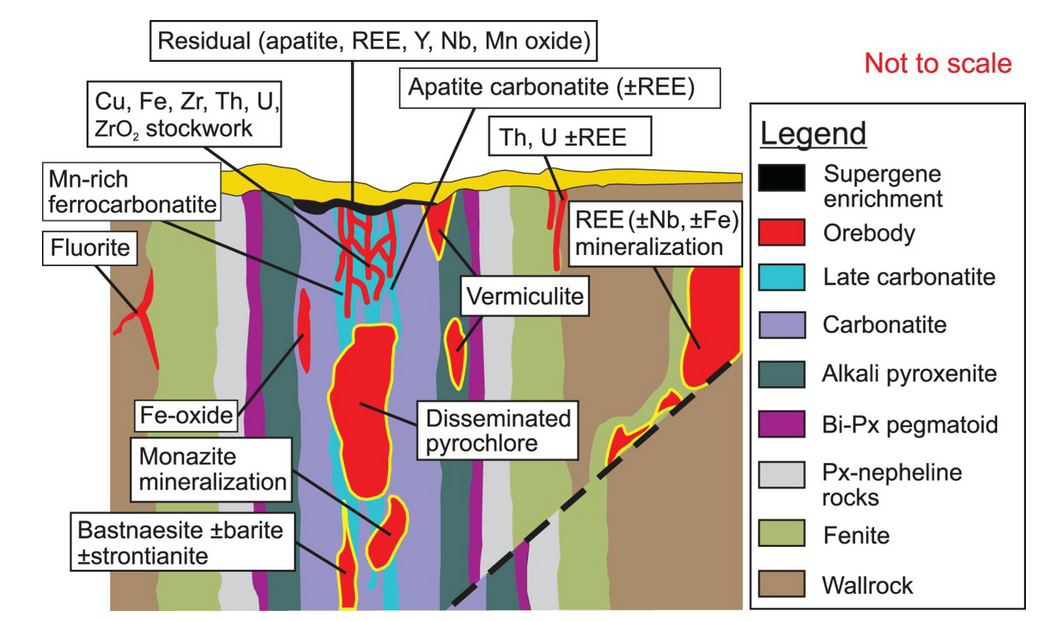
REE's in Carbonatites
2. Alkaline magmas are rare and unusually enriched in elements such as zirconium, niobium, strontium, barium, lithium, and rare earth elements. Their formation is complex and not fully understood but derived through small degrees of partial melting of rocks in the Earth's mantle. Further changes in response to variations in pressure, temperature, and composition of surrounding rocks result in mineral deposits quite diverse and awkward to classify
3. Placer Deposits - concentrations of heavy minerals in old and recent fluvial (river) systems or sand islands subject to coastal long-shore drift
4. Ion-adsorption clay deposits in southern China are the world’s primary source of heavy REEs.Thick clay accumulations that host low concentrations of REEs (from about.04 to 0.25 percent total REE oxides) form in tropical regionswith moderate to high rainfall through successive processes:
- REEs are leached by groundwater from granite bedrock;
- thick zones of clay-rich soils develop above the granites; and
- mobilized REEs become weakly fixed (by ion-adsorption) onto clays in the soils. Despite their low concentrations in REEs, the clay depositsof south China are economic because the REEs can be easily extracted from the clays with weak acids.
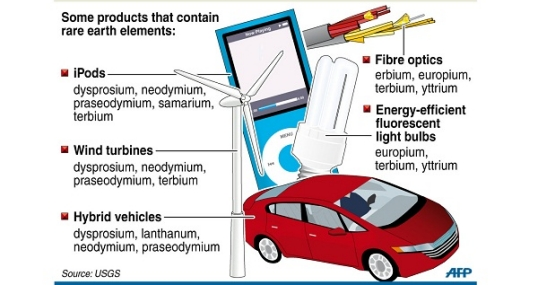
Common REE usus in Society
Some Australian Possibilities...
- Hydrothermally altered, pipe-like alkaline intrusions consisting of zirconium, hafnium, niobium and REE including yttrium. Typically concentrations of around @ 1.85% ZrO2, 0.04% HfO2, 0.44% Nb2O5, 0.03% Ta2O5, 0.14% Y2O3 and 0.74% TREO
- Peralkaline and alkaline granitic intrusions on the western margin of the Springdale Rift NSW Typical concentrations of about @ 1250 g/t ZrO2, 327 g/t REO, 146 g/t Y2O3, 45 g/t HfO2, 126 g/t Nb2O5, 54 g/t Ga2O3, 61 g/t ThO2, and 118 g/t Li2O14, hosted in deeply weathered and fresh leucogranite
- REE-phosphorus-uranium concentration in the Arunta Region of the Northern Territory about 135 km northwest of Alice Springs. The main REE-bearing minerals are fluorapatite, allanite and monazite. Possibly a hydrothermal stockwork vein-style deposit hosted in Paleoproterozoic metamorphosed igneous and sedimentary rocks, typically @ 3.2% TREO, 13% P2O5 with 26.15% of TREO being NdPr; Indicated Resources of 30 Mt @ 2.7% TREO, 12% P2O5 with 26.4% of TREO being NdPr and an Inferred Resource of 21 Mt @ 2.3% TREO, 10% P2O5 with 26.5% of TREO being NdPr
- Placer Deposits such as Monazite-xenotime mineralisation - monazite and xenotime occur in many of Australia’s heavy mineral sand deposits REEs in monazite and xenotime bearing alluvial sands northwest of Alice Springs. Basement rocks of the Arunta Block underlie the area. These are overlain by the mineralised Quaternary alluvial gravels and sands likely outwash fans derived from the older basement and which also have elevated grades of uranium and thorium. Grades are in the range of 300ppm TREO
- Ion-adsorption clay deposits One potential Australian clay-hosted area occursbetween Keith and Naracoorte SA, with some extension into Victoria, for deposits of mostly, praseodymium (pr), neodymium (Nd), terbium Tb)and dysprosium (Dy). Another possiblity lies in the recovery of REEs from tailings produced by bauxite(aluminum ore) mining, which could be considered a form of ion-adsorption clay deposit but needs investigation.
Processing of REEs
Rare earth elements-bearing mineral concentrates may contain many different REEs that must be further separated and refined. This required dozens of physical / chemical techniques and processes. Due to the required high degrees of concentration , deleterious radioactive Thorium and/or Uranium is an impurity that create problems both in handling and in waste mamagement.
The current average recovery rate in the industry is 50-60% .
- The traditional method of extracting pure REEs from mined material is called the solvent-exchange method, often involving ammonia-based solvents, , and consists of first crushing the rock into smaller chunks and then grinding it into a very fine dust. Unwanted materials (largely iron oxide minerals and carbonate minerals) are removed using various separation methods. What is left is an ore of REEs and radioactive material, which are then separated using additional means of chemical leaching.Rare earth elements are extremely similar chemically, which means they tend to stick together. Forcing them apart requires multiple sequential steps and a variety of powerful solvents to separate them one by one. Caustic sodium hydroxide causes cerium to drop out of the mix, for example. Other steps involve solutions containing organic molecules called ligands, which have a powerful thirst for metal atoms. The ligands can selectively bind to particular rare earth elements and pull them out of the mix.
- Bioleaching – using a variety of bacteria to remove different rare earth ions. The recovery rate is high but the process is slow, around 30 days. There is potential for in-situ leaching of unwanted minerals.
- Solid phase extraction involves crushing only to the stage of separating into individual mineral crystals then separating them by magnetic/non-magnetic properties or density (for example floating/sinking using heavy liquids like tetrabromoethane) or further separating the non-magnetic components by paramagnetic properites (using an extremely strong magnetic field to deflect a stream of crystals) or using AI to separate by size / colour / shape.
- Electrokinetic Method Chinese researchers are using electrical currents to free heavy rare earth elements — those with high atomic numbers like dysprosium and terbium — from ores. The new method creates an electric field above and below the soil, which improves the efficiency of the leaching so that lower amounts of chemicals are needed.
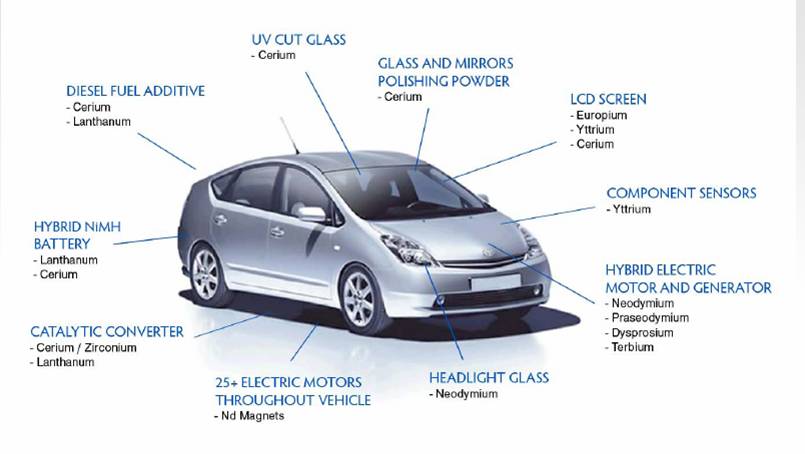
REEs in Cars
REE Economic Considerations
Because REE are generally found together, for many REEs, production
exceeds demand and will for the foreseeable future.Cerium is a good example of the fact that not all rare earth demand is equivalent.
When you produce Dysprosium, you are always producing much more cerium than Dysprosium. That doesn’t mean that there is a market for Cerium. In fact, it is more correct to say that some Cerium/Lanthanum/Neodymium deposits contain recoverable Dysprosium.
There are four or five critical REEs that each have individual markets. One of them is neodymium, because it’s the most important REE used in permanent magnets. The others are heavy rare earth elements (HREEs), including Europium, Terbium, Dysprosium and Yttrium. The latter isn’t really an REE, but it’s associated with them.
The big issue in magnets is the HREE (heavy rare earth element) Dysprosium.
There is not now, nor has there ever been, any production of Dysprosium from outside of China.
Another country of supply would be beneficial. Dysprosium is already in short supply.
Some hard rock Dysprosium is found in the Kimberley of Western Australia with the benefit of added Terbium – both marketable In a world of climate change the abundance and useful properties of by product Cerium for UV absorption, water purification, pigment and photo-sensitive glass could be the subject of innovative research.
For REEs it is pretty well a necessity to value add with on-shore processing.
Further value adding through the development and manufacture of small-size, low weight, high value REE containing components would certainly broaden of economic base of Australia.
Sources:
https://www.ga.gov.au/scientific-topics/minerals/mineral-resources-and-advice/australian-resource-reviews/rare-earth-elements REE map of Australiahttps://d28rz98at9flks.cloudfront.net/130434/130434_00_1.pdf
https://pubs.geoscienceworld.org/msa/elements/article/17/5/327/611057/Formation-of-Rare-Earth-Deposits-in-Carbonatites
https://www.tandfonline.com/doi/full/10.1080/25726838.2018.1516935 carbonatite REE deposits image
https://geology.com/usgs/ree-geology/
https://pubs.usgs.gov/fs/2014/3078/pdf/fs2014-3078.pdf
https://pubs.usgs.gov/sir/2011/5094/pdf/sir2011-5094.pdf
https://web.mit.edu/12.000/www/m2016/finalwebsite/solutions/greenrefining.html
https://americanresources.org/what-the-auto-industry-rare-earth-elements-have-in-common/ car image

