Oil Shale and Tar Sands
Oil Shale and Tar Sands

Oil shale
Formation of oil shale
Australian Deposits
Torbanite
Tasmanite
Toolebuc
Eastern Queensland
Refining the oil shale
Oil / Tar Sands
Oil Sand
Tar Sands

Oil Shale
Oil shale is a fine-grained sedimentary rock containing large amounts of organic matter (kerogen), which can yield substantial quantities of hydrocarbons.Oil shale is essentially a petroleum source rock which has not undergone the complete thermal maturation required to convert organic matter to oil.
In addition, the further geological processes of hydrocarbon migration and accumulation which produce conventional crude oil resources trapped in subsurface reservoirs has not occurred.
The unconventional shale oil resource can be transformed into liquid hydrocarbons by mining, crushing, heating, processing and refining, or by in situ heating, oil extraction and refining.
One tonne of commercial grade oil shale may yield from about 100 to 200 litres (L) of oil, that is approximately a half to one barrel of shale oil per tonne of oil shale.
Formation of oil shale
The process, in general, begins with the building of organic matter through the action of PHOTOSYNTHESIS. In the case of marine deposits, this begins with phytoplankton, which then enter the food chain that leads to the sedimentation of zooplankton rich in proteins, carbohydrates, and lipids. In terrestrial burial, the process begins in higher order organic life such as trees and shrubs, which then deposit primarily carbohydrates and lignin. The fraction of minerals to organic matter and the composition of the organics themselves lead to differences in the way that organic chemical reactions proceed and are catalyzed. As organic matter is buried, the process of diagenesis compacts it into organic rich sedimentary rocks. Further degrees of diagenesis then lead to the generation of kerogen which is then, through deeper burial and catagenesis, transformed through various means into crude oil and natural gas, or coal, depending on the precise conditions. In the case of oil shale and, according to some geologists, bitumen sands, the process of catagenesis is never allowed to be completed due to insufficient burial (temperatures and pressures too low).Diagenesis is a process of compaction under mild conditions of temperature and pressure. When organic aquatic sediments (proteins, lipids, carbohydrates) are deposited, they are very saturated with water and rich in minerals. Through chemical reaction, compaction, and microbial action during burial, water is forced out and proteins and carbohydrates break down to form new structures that comprise a waxy material known as “kerogen” and a black tar like substance called “bitumen”. All of this occurs within the first several hundred meters of burial.
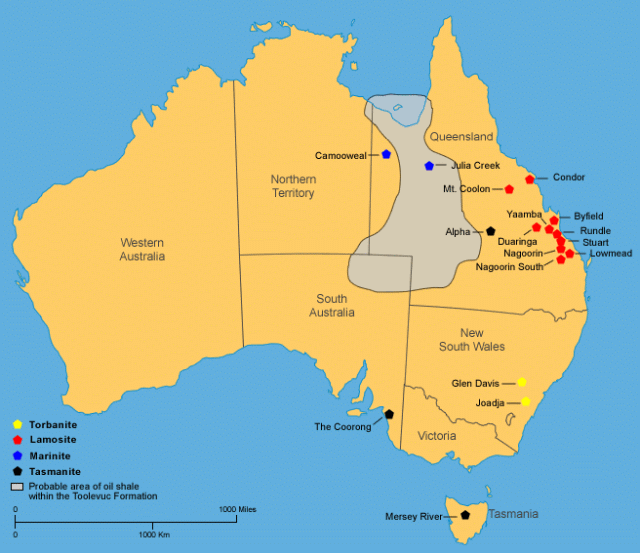
Australian Deposits
Australia has a large unconventional and currently non-producing identified shale oil resource of 131 600PJ (22 390mmbbl) which could potentially contribute to future oil supply if economic and environmental challenges can be overcome.The majority of Australian shale oil resources of commercial interest are located in Queensland, in the vicinity of Gladstone and Mackay.
Thick Cenozoic lacustrine oil shale deposits (lamosite) of commercial interest are predominantly in a series of narrow and deep extensional basins near Gladstone and Mackay. Oil shale deposits of varying quality also occur in New South Wales, Tasmania, and Western Australia in sedimentary sequences of Permian, Cretaceous and Cenozoic age.
The oil-shale deposits of Australia range from small and noneconomic to deposits large enough for commercial development.
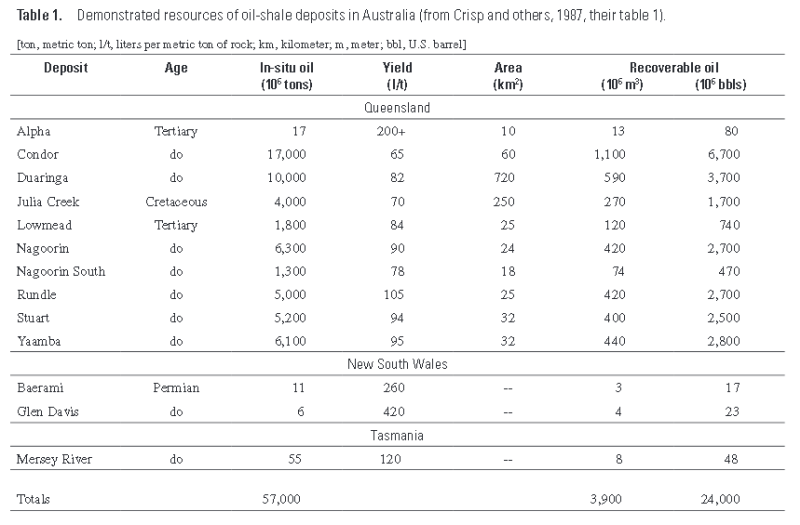
The “demonstrated” oil-shale resources of Australia total 58 billion tons, from which about 3.1 billion tons of oil (24 billion barrels) is recoverable.
Australian oil-shale deposits range in age from Cambrian to Tertiary and are diverse in origin.
The deposits are located in the eastern one-third of the country, including Western and Eastern Queensland, New South Wales, South Australia, Victoria, and Tasmania.
The deposits having the best potential for economic development are those located in Queensland and include the lacustrine Rundle, Stuart, and Condor deposits of Tertiary age.
The marine Toolebuc oil shale of Early Cretaceous age occu - pies a large area mostly in Queensland.
The torbanite deposits at Joadja Creek and Glen Davis in New South Wales and the tasmanite deposits in Tasmania were mined for shale oil in the last half of the 1800s and into the early 1900s.
The remaining resources of these high-grade deposits are not commercially important.
Torbanite
Much of the early production of oil shale in Australia was from the torbanite deposits of New South Wales. As many as 16 deposits were exploited between the 1860s and 1960s.During the early years of mining, torbanite was used for gas enrichment in Australia and overseas, but paraffin, kerosene, and wood preserving and lubricating oils were also produced.
Later, in the 1900s, torbanite was used to produce gasoline. Although the torbanite assayed as high as 480 to 600 l/t, the average feed to the retort was probably about 220 to 250 l/t. Of 30 deposits in New South Wales, were commercially exploited.
Two small deposits of torbanite have been investigated in Queensland. These include the small but high-grade Alpha deposit, which constitutes a potential in-situ resource of 19 million U.S. barrels and a smaller deposit at Carnarvon Creek.
Tasmanite
Several companies attempted to develop the marine tas - manite deposits of Permian age in Tasmania during the early 1900s.Between 1910 and 1932, a total of 1,100 m 3 (about 7,600 barrels) of shale oil was produced from several intermittent operations. Further developments are unlikely unless new resources are found.
Toolebuc Oil Shale
Oil shale in the marine Toolebuc Formation of Early Cretaceous age underlies about 484,000 km 2 in parts of the Eromanga and Carpenteria Basins in Queensland and adjacent States.The oil-shale zone ranges from 6.5 to 7.5 m in thickness but yields on average only about 37 l/t, making it a low-grade resource.
However, the Toolebuc Formation is estimated to contain 245 billion m 3 (~1.7 trillion barrels) of in-situ shale oil.
Excluding weathered oil shale from the surface to a depth of 50 m, about 20 percent (49 billion m 3 or 340 billion barrels) of the shale-oil resource between the depths of 50 to 200 m could be produced by open-pit mining.
The oil shale also contains potential resources of uranium and vanadium.
One of the more favorable localities for oil-shale development is near Julia Creek, where the Toolebuc oil shale is near the surface and is amenable to open-pit mining.
The resources of shale oil in the Toolebuc Formation suitable for open-pit mining total 1.5 bil - lion U.S. barrels, but the oil shale is too low grade for development at present
The organic matter of the Toolebuc oil shale is composed largely of bituminite, liptodetrinite, and lamalginite.
The atomic hydrogen to carbon (H/C) ratio of the organic matter is about 1.1 ±0.2 with high aromaticity (>50 percent). Only 25 percent of the organic matter converts to oil by conventional retorting .
Eastern Queensland
As a result of the increase in the price of crude oil related to the oil crisis of 1973–74, exploration for oil shale in Australia was greatly accelerated.Several companies identified or confirmed sizable resources of oil shale at Rundle, Condor, Duaringa, Stuart, Byfield, Mt. Coolon, Nagoorin, and Yaamba in eastern Queensland during the late 1970s and early 1980s.
However, by 1986, the prices of crude oil dropped dramati - cally, and interest in the exploitation of oil shale diminished.
Nine Tertiary oil-shale deposits in eastern Queensland have been investigated by exploratory core drilling—Byfield, Condor, Duaringa, Lowmead, Nagoorin, Nagoorin South, Rundle, Stuart, and Yaamba .
Most of these deposits are lamosites that were deposited in freshwater lakes located in grabens, commonly in association with coal-forming swamps.
The mineral fraction is typically composed of quartz and clay minerals with lesser amounts of siderite, carbonate minerals, and pyrite.
The sizes of the deposits range from 1 to 17.4 billion tons of in-situ shale oil with cutoff grades of around 50 l/t.
Three of the largest deposits are Condor (17.4 billion tons), Nagoorin (6.3 billion tons), and Rundle (5.0 billion tons) (Crisp and others, 1987).
The Stuart oil-shale deposit, estimated to contain 3 billion barrels of in-situ shale oil, is under development by the Southern Pacific Petroleum (SPP) and Central Pacific Minerals (CPM) companies.
As of February 2003, 1.16 million tons of oil shale were mined by open pit from which 702,000 barrels of shale oil were recovered by the Taciuk retorting process.
Taciuk Processor
Refining the oil shale
In-Situ oil shale retorting In the same manner that natural mineral catalysts help to transform kerogen to crude oil through the process of catagenesis, metal catalysts can help transform large hydrocarbons into smaller ones. The modern form of “catalytic cracking” utilizes hydrogen as catalyst, and is thus termed “hydrocracking”. This is a primary process used in modern petroleum refining to form more valuable lighter fuels from heavier ones. Oil that is produced from the refining of oil shale is typically referred to as synthetic crude oil, but the process is more closely allied with traditional crude oil refining than syn-fuel processes such as “gas to liquids”. The primary process is referred to as “retorting”, and involves the “cracking” (or destructive distillation or pyrolysis) of larger carbon chains into smaller ones in the absence of oxygen. Syn-fuel processes (such as Fisher-Tropsch) actually build up larger hydrocarbons from smaller ones, which is the opposite of cracking. Retorting is the cracking process used in shale oil refining, and first breaks down the kerogen to release hydrocarbons, and then further cracks the hydrocarbons into lower weight products. Retorting can occur in a traditional refining capacity, or may be conducted in-situ. In-situ processes require that the oil shale be heated, to release the petroleum liquids, prior to extraction from the ground. Oil shale distillates (products of retorting) typically favor the production of middle-distillates (diesel and kerosene), and have higher concentrations of nitrogen than crude oil. To produce light-distillates (such as gasoline) additional processing, such as hydrocracking, is required to break down the larger hydrocarbons. Also, the nitrogen must be removed through some hydrotreating process, comparable to hydrogen desulfurization to remove sulfur from crude oil, such as hydrodenitrogenation. Because hydrogen will be required in the refining process (bitumen is carbon rich and hydrogen poor), an additional source of hydrogen (such as methane) is needed. Therefore, some other fuel in addition to bitumen is needed to produce synthetic crude oil, which adds to the cost and energy intensity (and carbon dioxide emissions) of the process (2 fuels 1 fuel).
Oil / Tar Sands
Oil / tar sands are a mixture of sand, water, clay and bitumen.Bitumen is oil that is too heavy or thick to flow or be pumped without being diluted or heated.
At 10° C/50° F, bitumen is hard as a hockey puck. Some bitumen is found within 70 metres (200 feet) of the surface, but the majority is deeper underground.
Bitumen is so viscous that at room temperature it acts much like cold molasses.
A variety of treatment methods are currently available to oil sands producers and new methods are put into practice as more research is completed and new technology is developed.

Oil Sand
Oil sand can be found in several locations around the globe, including Venezuela, the United States and Russia, but the Athabasca deposit in Alberta is the largest, most developed and utilizes the most technologically advanced production processes.Alberta's oil sands lie under 142,000 km2 of land (54,800 mi2). Only about three per cent, or 4,800 km2 (1,850 mi2), of that land could ever be impacted by the mining method of extracting oil sands.
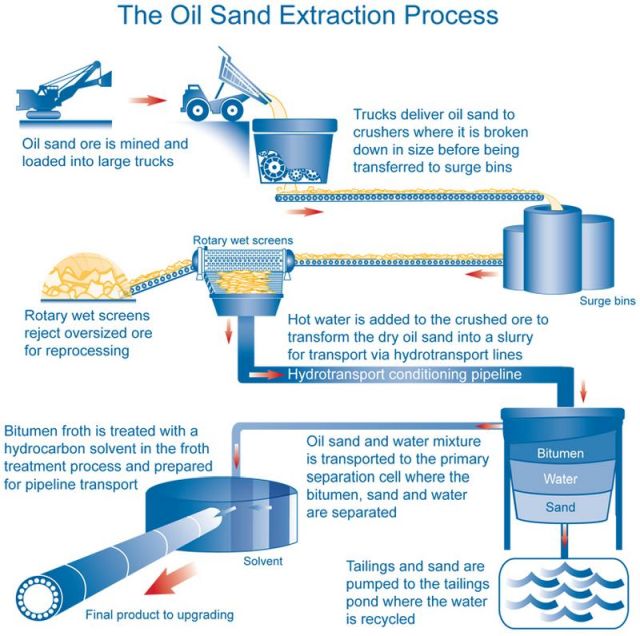
The remaining reserves that underlie 97 per cent of the oil sands surface area are recoverable only by in-situ (drilling) methods, which require very little surface land disturbance.
The oil sands area actively being mined is 904 km2 (346 mi2).

tar sands
Tar Sands
Tar sands (also known as oil sands) are a mixture of mostly sand, clay, water, and a thick, molasses-like substance called bitumen.Bitumen is made of hydrocarbons—the same molecules in liquid oil—and is used to produce gasoline and other petroleum products.
Extracting bitumen from tar sands—and refining it into products like gasoline—is significantly costlier and more difficult than extracting and refining liquid oil.
Common extraction methods include surface mining—where the extraction site is excavated—and “in-situ” mining, where steam is used to liquefy bitumen deep underground.
On a lifetime basis, a gallon of gasoline made from tar sands produces about 15% more carbon dioxide emissions than one made from conventional oil.
This important difference is attributable to the energy intensive extraction, upgrading, and refining process.
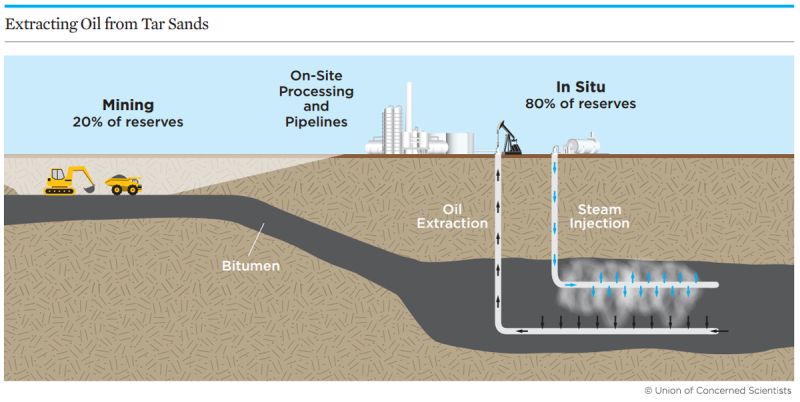
Unfortunately, the carbon emissions associated with extracting tar sands could increase over time, as in-situ mining—which creates more emissions than surface mining—is used to extract bitumen located deeper and deeper in the earth.
Tar sands also impact water supplies. For every gallon of gasoline produced by tar sands, about 5.9 gallons of freshwater are consumed during the extraction, upgrading, and refining process.
That’s roughly three times as much as used for conventional oil. Much of this water is polluted by toxic substances harmful to human health and the environment.
When surface mining is used, the wastewater ends up in toxic storage ponds. These ponds can cover over 30 square miles—making them some of the largest man-made structures on the planet.
When in-situ mining is used, wastewater is stored in the same well the bitumen is extracted from—risking contaminated groundwater if a leak occurs.

