Geochemistry and Ore Deposits
Geochemistry and Ore Deposits
Analytical chemistry
Natural processes
Geologists
Australia
How geologists use ore-deposit
models
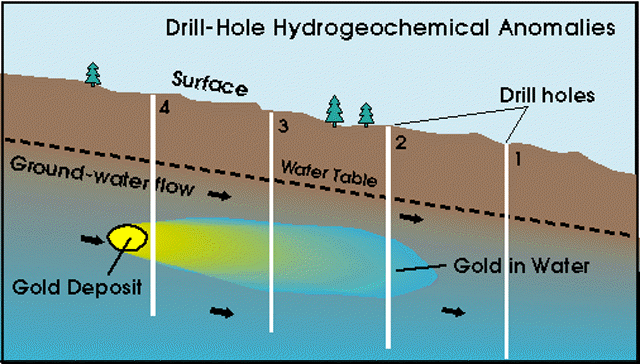
Detecting the trace
elements carried from a deposit by ground water
Analytical chemistry plays a key role in our continuing quest to understand how ore deposits formand in the practical exploration for ore deposits. If you pick up an ordinary rock that builds thecrust of the Earth and determine its chemical composition, for every billion atoms, 1 to 10,000atoms will be metallic elements such as gold, silver, platinum, mercury, copper, cobalt, nickel,chromium, lead, zinc, molybdenum, tin, and tungsten.
Natural processes in the Earth s crust have the remarkable ability to concentrate and purify certain rare metallic elements to form unusualdeposits of minerals that contain 1,000 to 10,000 times the amounts found in ordinary rocks.
With today's modern mining and extraction technology, it has become possible to mine verylow-grade deposits. For example, gold can be economically recovered from rocks that contain lessthan one tenth of an ounce of gold per ton of rock. But gold continues to be expensive because ofthe cost in locating the deposit, mining the rock, and extracting the small amount of gold in each tonof rock. All of the inorganic raw materials used to manufacture the products of todaystechnological society have to be either mined or recycled.
Almost every process that takes place in the Earth s crust, whether from the action of molten rock,heat and pressure at depth, hot springs or steam, running water, weather, or biological activity cancontribute to the formation of an ore deposit.

Geologists use the principles of chemistry to try tounderstand how these processes scavenge elements from ordinary rock, transport them, andconcentrate them to form an ore deposit.
Geologists have developed models that describe the physical characteristics and chemical composition of each ore deposit type and how they relate tothe geologic environment in which they form similar to the way biologists describe how an organismfits into a particular environmental niche. In Australia and many other parts of the world, almost all of the rich ore deposits exposed atthe surface have already been discovered. Most of the ore yet to be found is not visible to the human eye. Therefore, geologists have had to improve their understanding and develop moresophisticated ways to detect where ore deposits can occur.
How Geologists Detect Ore Deposits
Two main approaches are used to detect deposits hidden below the surface. One uses the ore-deposit model, and the other is based on the detection of a dispersion halo that extends forsome distance from the deposit.
The following analogy shows how geologists use ore-deposit models . If all but the tip of the tail ofan elephant was buried by a landslide, a biologist could recognize from the skin, hair, and shape ofthe appendage that the tail belonged to a mammal. With advanced testing of tissue samples, abiologist could prove that the tail belongs to an elephant and could easily predict that the bodyshould be buried about 1 meter below the tip of the tail.
Most ore-deposit models are not as advanced as biologists models for elephants, but a few arenearly so.
Several copper and molybdenum porphyry deposits, located as deep as 1500 metres below the surface, have been discovered based on small surface exposures measuring a few metres across. These exposures were of breccia pipes (vertical pipe-shaped bodies of pulverizedrock), which are known to extend hundreds of metres above the main body of porphyry deposits.
Because not all porphyries contain deposits of economic metals, geologists can collect and analyzefield samples to determine what metals the porphry will contain, and if it is worth drilling.
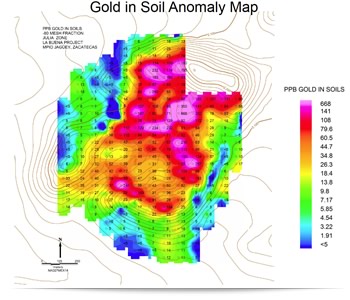
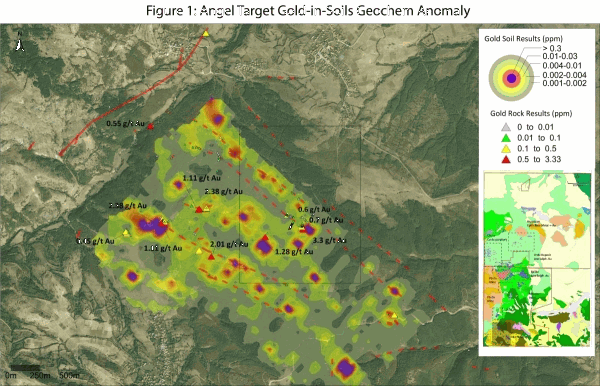
Mineral scavengers provide a clue
There is another way of detecting the trace elements carried from a deposit by ground water.
Ground water is drawn upward by evaporation at the surface. During this upward migration, traceelements in the water are affixed to minerals in the overburden. The affixation, or bonding, mayrange from weak to very strong. The strength of this bonding depends on the chemical nature ofboth the trace element and the host mineral. The differences in bond strength is comparable to thedifference between the weak electrostatic attraction that holds an inflated balloon to a wall and anail driven into a stud.
Minerals that are capable of scavenging trace elements from ground water with increasing bondstrength include hydrated aluminum silicates (clays), secondary carbonates, amorphous(noncrystalline) oxides of manganese, and the amorphous and crystalline oxides of iron. Traceelements scavenged by these minerals are removed by treating samples of overburden withchemicals that react selectively with each mineral phase. Sequential selective extractions are used torelease trace elements from the host minerals in the order of increasing bond strength such as claysfirst and crystalline iron oxides last.
The principal advantage of selective extractions is that they facilitate the distinction of elements thathave migrated from other sources from those normally present in the overburden. Thus thepresence of a gold deposit in Western Australia may well be indicated by the occurrence of gold, or itsassociated elements, arsenic and antimony, in a specific mineral phase in the overburden.

